| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Case Report
Volume 7, Number 4, December 2018, pages 63-68
A Molecular Study of Solid Pseudopapillary Neoplasm of the Pancreas in a Pediatric Patient
Vera Lucia Antunes Chagasa, Marlon Dias Mariano Santosb, Juliana de Saldanha da Gama Fischerb, Bruna dos Santos Paiva Ribeiroa, Fernando Colonna Rosmana, Paulo Costa Carvalhob, Danielle Fornyc, Maria da Gloria da Costa Carvalhoa, d
aDepartment of Pathology, Faculty of Medicine, Clementino Fraga Filho University Hospital, Federal University of Rio de Janeiro, Rua Rodolpho Paulo Rocco 255 - Ilha do Fundao - Rio de Janeiro - RJ 21941-590, Brazil
bComputational Mass Spectrometry & Proteomics Group, Carlos Chagas Institute, Fiocruz, Rua Professor Algacyr Munhoz Mader 2135-2261 - Cidade Industrial de Curitiba, Curitiba - PR 81310-020 Parana, Brazil
cInstituto de Puericultura e Pediatria Martagao Gesteira, Federal University of Rio de Janeiro, Rio de Janeir, Brazil
dCorresponding Author: Maria da Gloria da Costa Carvalho, Department of Pathology, Faculty of Medicine, Clementino Fraga Filho University Hospital, Federal University of Rio de Janeiro, Rua Rodolpho Paulo Rocco 255 - Ilha do Fundao - Rio de Janeiro - RJ 21941-590, Brazil
Manuscript submitted November 8, 2018, accepted December 12, 2018
Short title: Pediatric Solid Pseudopapillary Neoplasm
doi: https://doi.org/10.14740/ijcp320
| Abstract | ▴Top |
Solid pseudopapillary neoplasm of the pancreas (SPNP) is considered as a tumor with low malignant potential. Little is known about how its molecular heterogeneity is involved in its pathogenesis. We aim to evaluate tumor heterogeneity by immunohistochemistry (IH) and proteomics in three macroscopically distinct areas. Tumor fragments were obtained from a 12-year-old female patient. We identified by mass spectrometry (MS) 1,427, 5,786, and 4,298 proteins for each sample, 1,337 being common to all fragments. Several MS results were immunohistochemically validated. Our results demonstrate unique intra-tumor protein profiles based on its heterogeneity.
Keywords: Molecular study; Solid pseudopapillary neoplasm of the pancreas; Pediatric patient
| Introduction | ▴Top |
Intra-tumor heterogeneity has been recognized for a long time in different phenotypic features, such as cellular morphology, metabolism, proliferative and metastatic potential [1]. The intra-tumor phenotypic heterogeneity of solid pseudopapillary neoplasm of the pancreas (SPNP) is widely described in the literature [2-4], but it is poorly understood at a molecular level. It consists of a heterogeneous mixture of functionally distinct cells [4]. SPNP is a rare neoplasm of uncertain cell line, with both low potential for malignancy and excellent prognosis for most patients [5]. It accounts for about 1% to 3% of all exocrine pancreatic neoplasms [6]. SPNP occurs mainly, but not exclusively, in young women [7] including those in childhood and adolescence [8]. Its clinical course is prolonged and indolent, and the disease is usually asymptomatic; some signals and symptoms include the appearance of a palpable mass, abdominal pain and discomfort, and nausea [5]. SPNP is characterized by a solid-cystic growth, with pseudopapillary structures [5, 9]. Surgical resection is the treatment of choice and usually leads to a good prognosis, even if there are distant metastases or recurrence [10, 11]. Despite the diverse studies using electron microscopy and immunohistochemistry (IH), the cellular origin of this tumor remains uncertain, and it is hypothetically suggested that its ontogeny is of a multipotential primitive cell [12, 13]. Some authors [14] have suggested an extra-pancreatic origin due to the several reported cases of the primary tumor at different sites, such as ectopic pancreas [15], retroperitoneum [16], gastroduodenal area [17], and ovary [18, 19].
Neoplasia consists of a heterogeneous mixture of functionally distinct cells and the SPNP expresses a variety of immune markers in heterogeneity [4, 5]. Based on these reports we evaluated the molecular heterogeneity of this neoplasia. We used mass spectrometry (MS) to identify proteins in three different areas of the tumor and compared the results with the immunohistochemical panel. This is a rare tumor in a child, which until now has not been reported about a lot. Although this is a tumor of low malignancy, a recent study showed a methylation of P16 gene that can represent a potential malignancy [20]. As far as we know, this is the first study that compares the results of the proteomics analysis with the routine IH.
| Case Report | ▴Top |
This study was approved by the Research Ethics Committee of the Clementino Fraga Filho University Hospital (HUCFF) of the Federal University of Rio de Janeiro (UFRJ), with CAAE number 64915717.0.0000.5257. A 12-year-old female patient was admitted to HUCFF’s surgical ward complaining of nausea and pain in the right hypochondrium 1 month before admission to hospital. The patient resides at home with basic sanitation, along with her parents and two siblings. All are healthy and there are no reports of chronic diseases or neoplasms in the family. There is no report, in the medical records, of the use of medicines before the diagnosis.
The physical examination on admission revealed a palpable mass with a diameter of 10 cm in the right hypochondrium. The patient was eupneic and anicteric. The heart rate was 90 beats/min, the blood pressure was 102/60 mm Hg and the axillary temperature was 35.5 °C. Laboratory findings (complete blood count) showed red blood cells of 4.89 × 106/mm3, hemoglobin of 13.6 g/dL, hematocrit of 41%, leukocytes of 5,700/mm3 (0/0/0/1/61/32/4), and platelets of 219,000/mm3. Biochemistry showed sodium of 145 mEq/L, urea of 13 mg/dL, creatinine of 0.7 mg/dL, and acidic acid of 3.6 mg/dL. Hepatogram showed aspartate aminotransferase (ASAT) of 56 U/L, alanine aminotransferase (ALAT) of 135 U/L, alkaline phosphatase of 771 U/L, and gamma glutamyl transferase (GGT) of 286 U/L. On hospital admission, a computed tomography (CT) scan of the chest and abdomen was performed.
The CT of the abdomen showed a rounded and heterogeneous mass in the head of the pancreas, with 6.7 (transverse (T)) × 6.3 (anteroposterior (AP)) × 6.4 (longitudinal (L)) cm, suggestive of an SPNP (Fig. 1a). The patient was submitted to Whipple’s surgery and continued to be accompanied in the hospital outpatient clinic with no intercurrences. The surgical specimen was referred to the Pathology Anatomy Service of the hospital. After gross description, samples were collected from three distinct areas from fresh tumor. Then the surgical specimen was immersed in 10% buffered formalin for further histopathological study (Fig. 1b). The patient continues to be regularly monitored at the hospital every 3 months by the outpatient clinics of Surgery, Pediatric Gastroenterology and Nutrology and has not presented any intercurrences so far. Pancreatin (CreonR) is used four times a day. The last laboratory examination (complete blood count) evidenced red blood cells of 4.56 × 106/mm3, hemoglobin of 12.3 g/dL, hematocrit of 36.8%, leukocytes of 4,130/mm3 (1.2/2.2/-/52.8/34.6/9.2), and platelets of 255,000/mm3. Hepatogram showed ASAT of 30 U/dL, ALAT: 24 U/dL, alkaline phosphatase, 100 U/L, and GGT of 14 U/L.
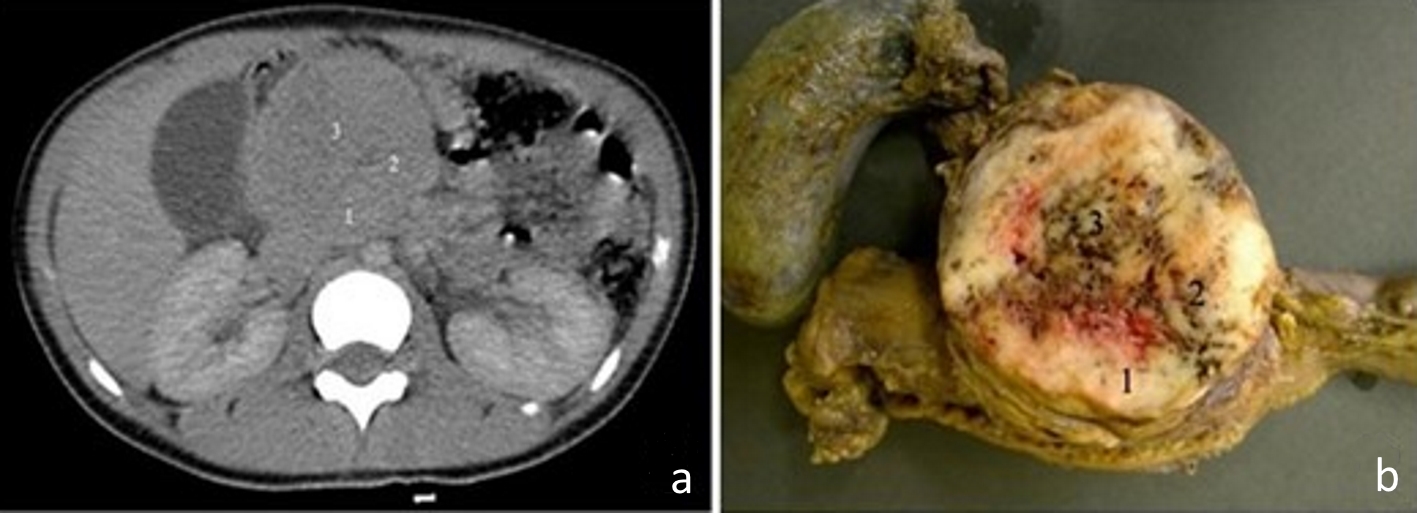 Click for large image | Figure 1. (a) Computed tomography image of the abdomen, in the portal phase, showing a rounded and heterogeneous mass in the head of the pancreas, measuring 6.7 cm (transverse) × 6.3 cm (anteroposterior) × 6.4 cm (longitudinal). Areas 1, 2 and 3 are the topographical areas where fresh tissue samples were collected for the molecular study. (b) Transverse section of solid pseudopapillary neoplasm in the head of the pancreas presenting a solid mass with heterogeneous and whitish aspects, associated with blackened hemorrhagic areas. The numbers represent the regions where fresh tissue samples were collected for the molecular study: 1. peripheral; 2. intermediate; and 3. central. |
By macroscopy, we observed, in the head of the pancreas, a rounded mass measuring approximately 6.0 cm in diameter with a firm and elastic consistency, apparently encapsulated. The sections were whitish in color, with peripheral solid areas, and sometimes granular or otherwise softened areas, associated with hemorrhage ones (Fig. 1b).
By conventional microscopy, histopathological examination of hematoxylin and eosin-stained histological sections revealed neoplasia consisting of eosinophilic (Fig. 2a) or light cytoplasmic cells (Fig. 2b) with rounded or ovoid nuclei, with regular or slightly grooved contours, with uniform chromatin, occasional nucleoli, and about six mitoses in 10 chromogranin A (CgA). These cells were polyhedral, without cohesion, constituting cell masses. They were often arranged perpendicularly around a delicate, fibroconjunctive axis, forming a pseudopapillary aspect, at which time the cytoplasm appeared more elongated and the nuclei were located at the apical cellular border (Fig. 2c). Hemorrhagic areas were also observed. The neoplasia was isolated from the pancreas by fibrous band and the adjacent pancreatic parenchyma did not present significant histological alterations.
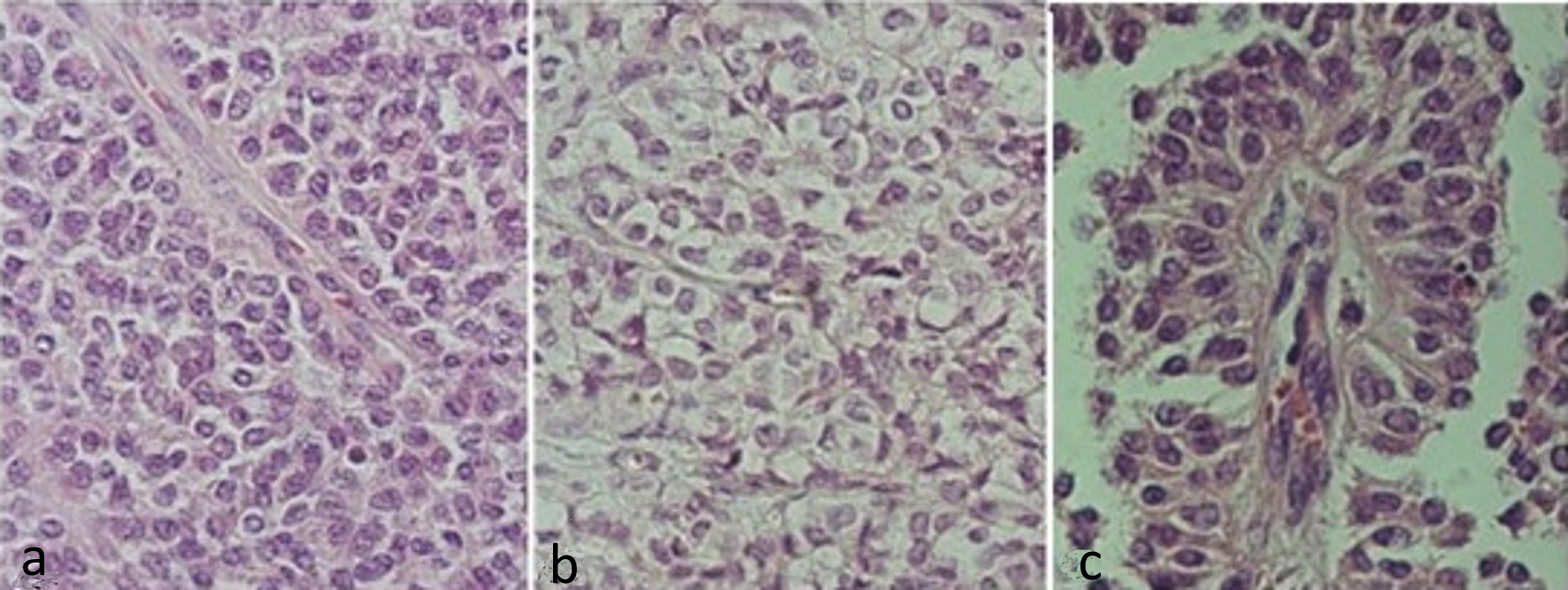 Click for large image | Figure 2. Hematoxylin and eosin stained slides of solid pseudopapillary neoplasm of the pancreas. (a) Solid pattern with barely cohesive cells presenting ovoid or rounded nuclei, sometimes grooved and eosinophilic cytoplasm. (b) Solid pattern showing cells with clear cytoplasm. (c) Pseudopapillary pattern, with cells distributed around vascular connective axis being the nuclei arranged in apical portion. |
In terms of proteomics, all three samples were pulverized with liquid nitrogen and then leased by immersion in a solution with 0.2% of RapiGest™ (w/v) in 50 mM triethylammonium bicarbonate [21]. The validation of peptide-spectrum matching (PSM) and label-free quantification analysis were conducted as previously described [22, 23]. Our proteomics results identified 34,173 mass spectra mapping to 7,321 peptides leading to the inference of up to 7,663 proteins, being 1,629 proteins according to maximum parsimony and 613 having at least one proteotypic peptide. Figure 3 shows a Venn diagram demonstrating the uniquely identified proteins according to each biological sample; it only considered proteins identified in at least two technical replicates. The analysis of proteins localized in tumor fragment 1 disclosed 1,427 proteins (17 unique), 5,786 in fragment 2 (1,775 unique) and 4,298 in fragment 3 (318 unique). A list of the proteins corresponding to each of the diagram’s area according to tumor location are available in the Supplementary 1 (www.theijcp.org).
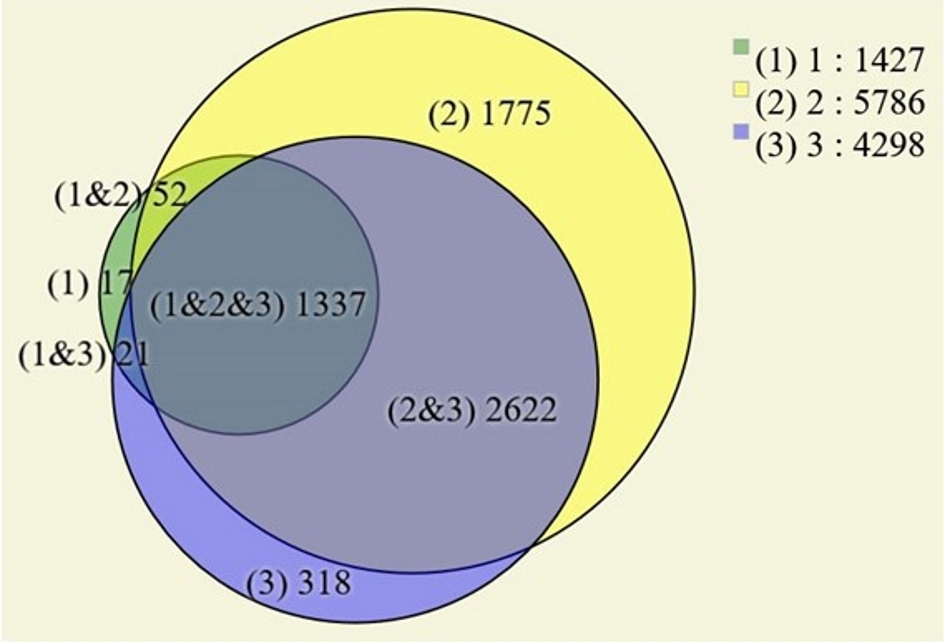 Click for large image | Figure 3. Venn diagram of the identified proteins in the three tumor areas. Only proteins found in two or more technical replicates were considered. |
MS validation by IH
The immunohistochemical assessment was positive to the antibodies against β-catenin, CD99, CD10, CD56, progesterone receptor, and Ki67. Reactions with anti-synaptophysin, anti-E-cadherin and CgA were negative. β-catenin expression was observed in nuclear and cytoplasmic location, with irregular cell distribution in the three fragments examined, the progesterone receptor was nuclear diffusely, and the CD10 was expressed with diffuse cytoplasmic granular pattern and the CD56 with apical membrane expression. The proliferation index by nuclear Ki67 was about 8%. CD99 antibody was negative in blocks 1 and 3 and positive in block 2, with a typical perinuclear granular pattern for this tumor (Fig. 4).
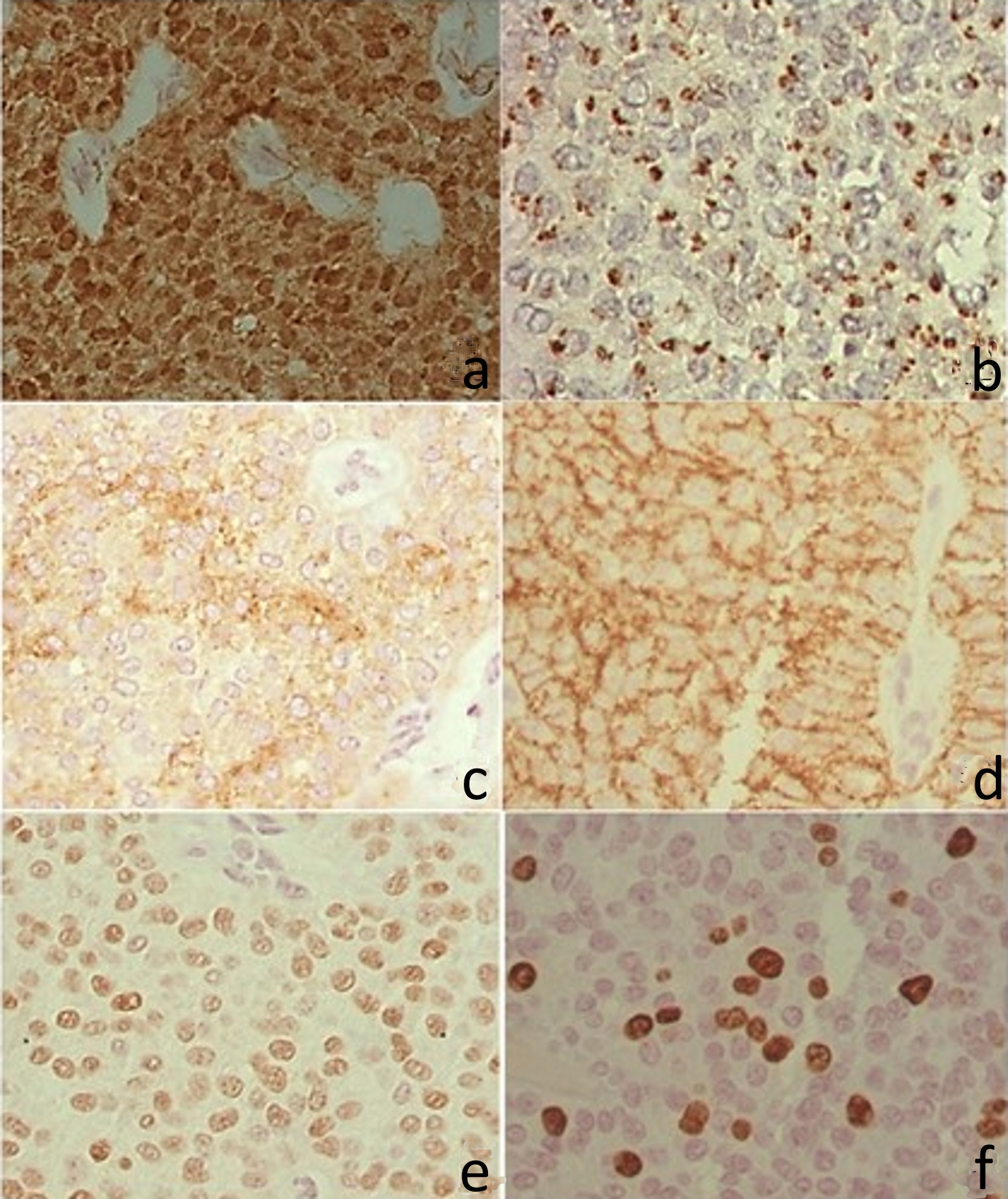 Click for large image | Figure 4. Immunohistochemistry. (a) Positive β-catenin in the nucleus and cytoplasm. (b) CD99 with perinuclear granular marking (dot). (c) CD10 with irregular positivity in the cytoplasm. (d) CD56 with cytoplasmic membrane positivity. (e) Progesterone receptor, with intranuclear labeling. (f) Ki67 with intranuclear labeling. |
Comparison between IH results and those obtained by MS
Immunohistochemical and MS analysis were both negative for CgA. β-catenin was positive in both IH and MS. CD99 was positive in both fragments labeled as 2 for MS and IH. CD10 and progesterone receptor were only positive in IH. CD56 was only negative in fragment 1 for MS. E-cadherin was only positive by proteomics for fragments 2 and 3. Synaptophysin was only negative in fragment 1 for MS and in the three fragments in IH. The results are represented in Table 1.
 Click to view | Table 1. Comparison Between Immunohistochemical Results and Those Obtained by Mass Spectrometry |
| Discussion | ▴Top |
We reported a pediatric case of SPNP removed by partial pancreatectomy from a female 12-year-old patient, where heterogeneity was found on the macroscopy. A molecular analysis was done, comparing the IH with the proteomics.
The intra-tumor heterogeneity is recognized as a process with great clinical importance and is related to pathological observation of different tumoral areas [24] associated with cancer progression, resistance to therapy, and recurrences [24, 25]. In our study, we observed the heterogeneous morphological aspect of SPNP by gross and histopathological examinations. The expression of different antibodies that identify several cell lines associated with differentiated immunoreactivity of one antibody (CD99), in relation to the three paraffin blocks used in the immunohistochemical reactions, suggests that there is also a heterogeneity of this neoplasm from the point of view of its immunophenotyping markers.
This fact demonstrates the importance of immunohistochemical analysis of more than one tumor fragment. CD99 paranuclear dot-like immunostaining is a key immunohistochemical staining feature for diagnosis of SNP and could be used to differentiate SPPT from other pancreatic tumors [9]. Although the reactivity to the CD99 was not uniformly observed among the blocks examined, it was not possible to compare this aspect with other reports, since no similar approach was found in the literature in relation to the study in different areas of the same tumor. A recent report emphasizes the importance of histopathological and molecular analysis of different fragments of the same tumor to obtain a better detection of intra-tumoral heterogeneity [26]. Some authors have used this system in the evaluation of tumors of the urinary bladder, stomach and large bowel [24]. However, for pancreatic neoplasms and especially for SPPN, no reports were found.
Proteomics revealed proteins common to the three fragments and several uniquely identified proteins in each fragment. β-catenin was identified by both proteomics and immunochemistry. Mutations in exon 3 of the β-catenin gene are observed in most SPNPs [27-29]. These mutations interfere with the degradation of the β-catenin protein that accumulates in neoplastic cells and is translocated to the nucleus where it increases the transcription of c-myc and cyclin D1 [27, 29], resulting in abnormal nuclear accumulation observed by IH in most SPNPs. Dysregulation of β-catenin/E-cadherin complex may be responsible for the lack of cell cohesion resulting in the typical cystic and pseudopapillary appearance of this neoplasm [30]. The aberrant E-cadherin expression is dependent of the cellular domain recognized by the antibody utilized: immunonegativity (extra-cellular domain) or nuclear reactivity (cytoplasmic domain) [31]. In our study we used antibody anti-E-cadherin that recognizes the extra-cellular domain, which could explain our results, where this protein was identified only as positive by MS in fragments 2 and 3. CD99, with a perinuclear granular pattern, is considered to be one of the main markers in the diagnosis of SPNP [27-29]; however, its positivity was observed in only one of the blocks by IH and MS. CD56, with immunoreactivity in the three blocks submitted to IH, was identified in the MS in two of the fragments. The progesterone receptor, present in the three fragments to IH, was identified by MS in two of the fragments, not as a nuclear receptor, but as membrane-associated progesterone receptors 1 and 2 [32-34], associated with a noncanonical pathway of that receptor. Different studies have examined the membrane-associated progesterone receptor in male and female reproductive tracts, the liver, neuroendocrine tissues, the immune system and breast and ovarian cancer [35]. However, as far as we know, there are no reports about SPNP. CD10 also detected in the three fragments submitted to the IH was not observed by MS. We presume that proteins undetected by MS, but verified with IH, are lowly abundant and thus insufficient for detection by MS when in a complex protein mixture. Another possibility is that the heterogeneous aspect of the tumor is responsible for the deficiency of a certain protein, considering the small size of the fragment obtained for molecular analysis. In conclusion, as far as we know, this is the first molecular case study report comparing different areas of the same tumor by IH and MS and showing the SPNP molecular and immunohistochemical heterogeneity.
SPNP is a rare tumor in children [36]. Lee et al demonstrated different clinical features in adults and children [37]. The study of this case can lead to a personal therapy for the disease, since we can find patterns of the protein expression in children. Also, with further studies it is possible to find a marker for possible malignancy and to develop preventive treatments.
Acknowledgments
The authors acknowledge the Fiocruz Parana mass spectrometry core facility for running the samples. This work was supported by National Council for Scientific and Technological Development (CNPq).
Conflict of Interest
The authors declare no conflict of interest.
Abbreviations
SPNP: solid pseudopapillary neoplasm of the pancreas; ASAT: aspartate aminotransferase; ALAT: alanine aminotransferase; CT: computed tomography; GGT: gamma glutamyl transferase; CgA: chromogranin A; PSM: peptide-spectrum matching; IH: immunohistochemistry; MS: mass spectrometry; T: transverse; AP: anteroposterior; L: longitudinal
| References | ▴Top |
- Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44(6):2259-2265.
pubmed - Hackeng WM, Hruban RH, Offerhaus GJ, Brosens LA. Surgical and molecular pathology of pancreatic neoplasms. Diagn Pathol. 2016;11(1):47.
doi pubmed - Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP. 2006;7(1):131-136.
pubmed - Ye J, Ma M, Cheng D, Yuan F, Deng X, Zhan Q, Shen B, et al. Solid-pseudopapillary tumor of the pancreas: clinical features, pathological characteristics, and origin. J Surg Oncol. 2012;106(6):728-735.
doi pubmed - Dinarvand P, Lai J. Solid Pseudopapillary Neoplasm of the Pancreas: A Rare Entity With Unique Features. Arch Pathol Lab Med. 2017;141(7):990-995.
doi pubmed - J AC, Lozano MD, Rotellar F, Marti P, Pedano N, Arredondo J, Bellver M, et al. Solid pseudopapillary tumor of the pancreas (SPPT). Still an unsolved enigma. Rev Esp Enferm Dig. 2010;102(12):722-728.
pubmed - Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9(1):35-40.
doi pubmed - Park M, Koh KN, Kim BE, Im HJ, Kim DY, Seo JJ. Pancreatic neoplasms in childhood and adolescence. J Pediatr Hematol Oncol. 2011;33(4):295-300.
doi pubmed - Guo Y, Yuan F, Deng H, Wang HF, Jin XL, Xiao JC. Paranuclear dot-like immunostaining for CD99: a unique staining pattern for diagnosing solid-pseudopapillary neoplasm of the pancreas. Am J Surg Pathol. 2011;35(6):799-806.
doi pubmed - Igbinosa O. Pseudopapillary tumor of the pancreas. An algorithmic approach. JOP. 2011;12(3):262-265.
pubmed - Nguyen NQ, Johns AL, Gill AJ, Ring N, Chang DK, Clarkson A, Merrett ND, et al. Clinical and immunohistochemical features of 34 solid pseudopapillary tumors of the pancreas. J Gastroenterol Hepatol. 2011;26(2):267-274.
doi pubmed - Balercia G, Zamboni G, Bogina G, Mariuzzi GM. Solid-cystic tumor of the pancreas. An extensive ultrastructural study of fourteen cases. J Submicrosc Cytol Pathol. 1995;27(3):331-340.
pubmed - Yamaguchi K, Miyagahara T, Tsuneyoshi M, Enjoji M, Horie A, Nakayama I, Tsuda N, et al. Papillary cystic tumor of the pancreas: an immunohistochemical and ultrastructural study of 14 patients. Jpn J Clin Oncol. 1989;19(2):102-111.
pubmed - Kosmahl M, Seada LS, Janig U, Harms D, Kloppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. 2000;436(5):473-480.
doi pubmed - Khaniya S, Shakya VC, Koirala R. Solid pseudopapillary tumor in an ectopic pancreas: an unusual presentation. J Surg Case Rep. 2017;2017(3):rjx050.
doi pubmed - Zhu H, Xia D, Wang B, Meng H. Extrapancreatic solid pseudopapillary neoplasm: Report of a case of primary retroperitoneal origin and review of the literature. Oncol Lett. 2013;5(5):1501-1504.
doi pubmed - Walter T, Hommell-Fontaine J, Hervieu V, Adham M, Poncet G, Dumortier J, Lombard-Bohas C, et al. Primary malignant solid pseudopapillary tumors of the gastroduodenal area. Clin Res Hepatol Gastroenterol. 2011;35(3):227-233.
doi pubmed - Gahlot GP, Mridha AR, Sable M, Sharma MC, Pramanik R, Kumar L. Solid pseudopapillary neoplasm of the ovary with metastases to the omentum and regional lymph nodes. Indian J Pathol Microbiol. 2016;59(3):348-350.
doi pubmed - He S, Yang X, Zhou P, Cheng Y, Sun Q. Solid pseudopapillary tumor: an invasive case report of primary ovarian origin and review of the literature. Int J Clin Exp Pathol. 2015;8(7):8645-8649.
pubmed - Chagas VLA, Ribeiro BSP, da Mota e Silva MS, Forny DN, Rosman FC, da Costa Carvalho MG. Epigenetics of solid pseudopapillary neoplasm of the pancreas. JOP. J Pancreas. 2018;19(4):223-227.
- Aquino PF, Fischer JS, Neves-Ferreira AG, Perales J, Domont GB, Araujo GD, Barbosa VC, et al. Are gastric cancer resection margin proteomic profiles more similar to those from controls or tumors? J Proteome Res. 2012;11(12):5836-5842.
doi pubmed - Carvalho PC, Fischer JS, Xu T, Cociorva D, Balbuena TS, Valente RH, Perales J, et al. Search engine processor: Filtering and organizing peptide spectrum matches. Proteomics. 2012;12(7):944-949.
doi pubmed - Carvalho PC, Lima DB, Leprevost FV, Santos MD, Fischer JS, Aquino PF, Moresco JJ, et al. Integrated analysis of shotgun proteomic data with PatternLab for proteomics 4.0. Nat Protoc. 2016;11(1):102-117.
doi pubmed - Cortes JM, de Petris G, Lopez JI. Detection of intratumor heterogeneity in modern pathology: a multisite tumor sampling perspective. Front Med (Lausanne). 2017;4:25.
doi - Stanta G, Bonin S. Overview on clinical relevance of intra-tumor heterogeneity. Front Med (Lausanne). 2018;5:85.
doi pubmed - Lopez JI, Cortes JM. Multisite tumor sampling: a new tumor selection method to enhance intratumor heterogeneity detection. Hum Pathol. 2017;64:1-6.
doi pubmed - Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, Cameron JL, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160(4):1361-1369.
doi - Shi C, Daniels JA, Hruban RH. Molecular characterization of pancreatic neoplasms. Adv Anat Pathol. 2008;15(4):185-195.
doi pubmed - Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, Nagahara N, et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61(23):8401-8404.
pubmed - Tang WW, Stelter AA, French S, Shen S, Qiu S, Venegas R, Wen J, et al. Loss of cell-adhesion molecule complexes in solid pseudopapillary tumor of pancreas. Mod Pathol. 2007;20(5):509-513.
doi pubmed - Chetty R, Serra S. Membrane loss and aberrant nuclear localization of E-cadherin are consistent features of solid pseudopapillary tumour of the pancreas. An immunohistochemical study using two antibodies recognizing different domains of the E-cadherin molecule. Histopathology. 2008;52(3):325-330.
doi pubmed - Aizen J, Pang Y, Harris C, Converse A, Zhu Y, Aguirre MA, Thomas P. Roles of progesterone receptor membrane component 1 and membrane progestin receptor alpha in regulation of zebrafish oocyte maturation. Gen Comp Endocrinol. 2018;263:51-61.
doi pubmed - Ryu CS, Klein K, Zanger UM. Membrane associated progesterone receptors: promiscuous proteins with pleiotropic functions - focus on interactions with cytochromes P450. Front Pharmacol. 2017;8:159.
doi pubmed - Tsai HW, Ho CL, Cheng SW, Lin YJ, Chen CC, Cheng PN, Yen CJ, et al. Progesterone receptor membrane component 1 as a potential prognostic biomarker for hepatocellular carcinoma. World J Gastroenterol. 2018;24(10):1152-1166.
doi pubmed - Dressing GE, Goldberg JE, Charles NJ, Schwertfeger KL, Lange CA. Membrane progesterone receptor expression in mammalian tissues: a review of regulation and physiological implications. Steroids. 2011;76(1-2):11-17.
doi pubmed - Leraas HJ, Kim J, Sun Z, Ezekian B, Gulack BC, Reed CR, Tracy ET. Solid Pseudopapillary neoplasm of the pancreas in children and adults: a national study of 369 patients. J Pediatr Hematol Oncol. 2018;40(4):e233-e236.
doi pubmed - Lee SE, Jang JY, Hwang DW, Park KW, Kim SW. Clinical features and outcome of solid pseudopapillary neoplasm: differences between adults and children. Arch Surg. 2008;143(12):1218-1221.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
