| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Original Article
Volume 13, Number 3, December 2024, pages 69-72
Accuracy of the Set Tidal Volume During Intraoperative Anesthetic Care: An In Vitro Evaluation
Jennifer Sawyera, Kelly Moonb, Michael Tobiasb, Julie Rice-Weimerb, Joseph D. Tobiasb, c, d
aHeritage College of Osteopathic Medicine - Dublin Campus, Dublin, Ohio and Ohio University, Athens, OH, USA
bDepartment of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH, USA
cDepartment of Anesthesiology & Pain Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
dCorresponding Author: Joseph D. Tobias, Department of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH 43205, USA
Manuscript submitted September 30, 2024, accepted November 18, 2024, published online December 2, 2024
Short title: Accuracy of Tidal Volume
doi: https://doi.org/10.14740/ijcp551
| Abstract | ▴Top |
Background: Precise adjustment of tidal volume (Vt) and minute ventilation remains a key component of intraoperative care. Control of Vt is regulated by internal pneumotachometers and flow meters, which may be separated from the patient by the anesthesia circuit and the internal circuitry of the anesthesia machine. Given this arrangement, there may be variations in delivered and exhaled (measured) Vt depending on the site of measurement.
Methods: The current study used an in vitro model to determine variations in inspired and expired Vt during various volume- and pressure-controlled modes of ventilation using an Avance CS2 anesthesia machine.
Results: During in vitro mechanical ventilation using pressure limited (15 and 20 cm H2O) and volume limited (Vt 50, 100, 200, and 300 mL), we saw slight discrepancies between Vt measured using a pneumotachometer placed between the endotracheal tube (ETT) and the anesthesia circuit as compared to those measured internally by the anesthesia machine.
Conclusions: Although the differences were statistically significant, the variations were no more than 5-6% at most at the higher Vt with either volume- or pressure-limited ventilation. These differences are unlikely to be clinically significant, thereby demonstrating the accuracy and safety of anesthesia machines from the modern era.
Keywords: Mechanical ventilation; Intraoperative ventilation; Tidal volume; Pneumotachometer
| Introduction | ▴Top |
The accurate control of tidal volume (Vt) is one of the key elements in limiting the risk of ventilator-induced lung injury, ensuring adequate ventilation, and improving outcomes in mechanically ventilated patients [1, 2]. In general, intraoperative mechanical ventilation can be delivered via one of two distinct methods: volume-controlled ventilation (VCV) or pressure-controlled ventilation (PCV). Especially in smaller patients, PCV is frequently chosen due to concerns with the inaccuracy of Vt during VCV [2]. Excessive ventilation with larger Vt may result in higher peak inflating pressures (PIPs) and the potential for barotrauma or volutrauma when the Vt is inadvertently larger than expected, while low Vt may result in atelectasis, hypoventilation, and hypocarbia.
During intraoperative anesthesia care using a circle system, the volume delivered by the ventilator into the circuit is not the same volume that reaches the patient. The fresh gas flow and the compliance of the circuit impact the Vt. Furthermore, it may be difficult to determine the volume delivered to the patient, since exhaled Vt is typically measured at the expiratory valve. In that location, the flow sensor measures exhaled gas plus the gas compressed in the circuit during the previous inspiration and therefore overestimates the delivered Vt [3-5]. The purpose of the current study was to determine the accuracy of the set Vt on the Avance CS2 Anesthesia Machine (GE Healthcare, Madison, WI) during intraoperative mechanical ventilation.
| Materials and Methods | ▴Top |
This prospective clinical study was conducted at Nationwide Children’s Hospital, Columbus, Ohio. It was approved by the Institutional Review Board of Nationwide Children’s Hospital, Columbus, Ohio (STUDY00003946). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. The study was registered with clinicaltrials.gov (identifier number NCT06232915) on January 31, 2024. As this was only an in vitro study that did not include patients, no informed consent was required. The study included an in vitro evaluation of the function of the Avance CS2 Anesthesia Machine (GE Healthcare, Madison, WI). The in vitro evaluation was performed using a lung analogue to compare the spirometry function of the machine with that of a separate pneumotachometer placed at the site where the endotracheal tube (ETT) connected to the anesthesia circuit (Fig. 1). Prior to data collection, a standard machine check was performed. Compliance and resistance compensations were performed during the machine check using a standard anesthesia circuit.
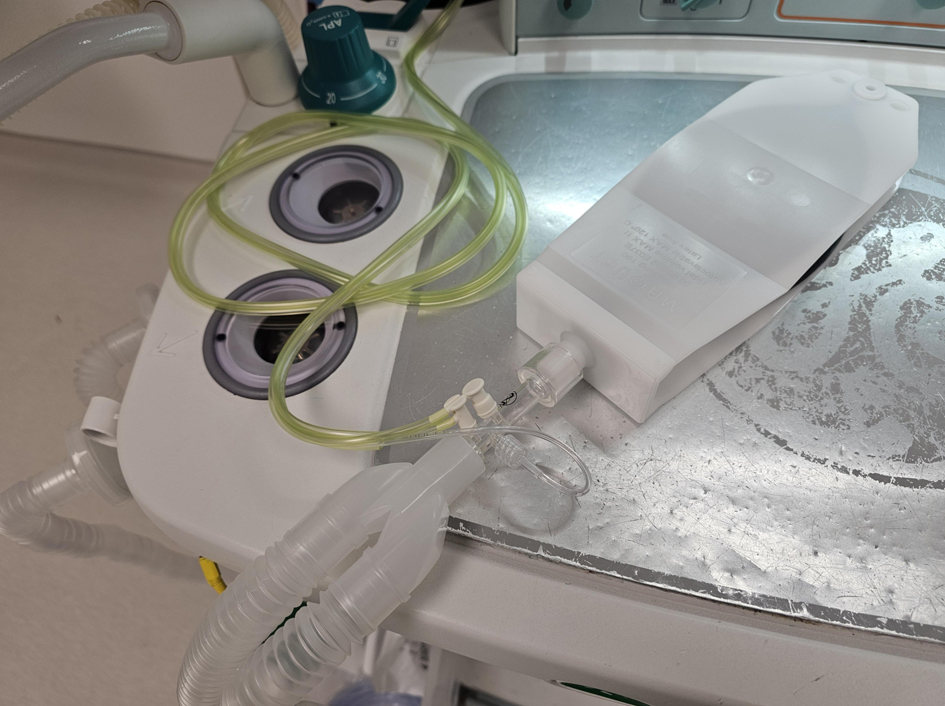 Click for large image | Figure 1. Photograph of the lung analogue device used in the current study. The pneumotachometer is attached between the anesthesia circuit and the 15 mm attachment to the lung analogue device. |
Data collection measured from the anesthesia machine included the set Vt as well as inspiratory and expiratory Vt using the internal spirometry of the anesthesia machine. Additionally, inspiratory and expiratory Vt were measured with a pneumotachometer (GE Pedi-lite spirometer, GE Healthcare, Madison, WI) placed between the anesthesia circuit and the ETT. Data were collected during six simulated ventilator settings including Vt of 50, 100, 200, and 300 mL as well as PIP of 15 and 20 cm H2O. Vt values were recorded (mL) for a total of 250 breaths for each of the six ventilator settings. Other ventilator parameters were kept constant: fraction of inspired oxygen concentration (FiO2) 40%, positive end expiratory pressure (PEEP) 5 cm H2O, inspiratory time 1 s, and respiratory rate 15 breaths/min. Data were collected from a total of 250 simulated breaths and recorded onto an Excel spreadsheet.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Vt were evaluated using the set Vt as the standard and comparing the inspiratory and expiratory Vt measured from the machine and the pneumotachometer to the set Vt. Additionally, the inspiratory Vt and the expiratory Vt from the machine and the pneumotachometer were compared to each other to evaluate the accuracy of the machine-based measurements. These values were compared using paired t-tests and analysis of variance with P < 0.05 considered as statistically significant.
| Results | ▴Top |
The results of the inspiratory Vt from the 250 in vitro measurements are outlined in Table 1. The data are presented as the mean ± SD. Each value is representative of 250 breaths. Patient mode volumes were measured by the pneumotachometer placed between the 15 mm adaptor of the ETT and the ventilator circuit. Volumes listed as ventilator mode were measured internally by the anesthesia machine using standard internal flowmeters and pneumotachometers. Regardless of the mode of ventilation (volume or pressure limited and the set Vt or PIP), the inspiratory and expiratory Vt were essentially the same from breath to breath as demonstrated by SDs of 0.4 - 0.8 for all values.
 Click to view | Table 1. Recorded Inspiratory Vt |
Although the differences were statistically significant for all comparisons (patient mode versus ventilator mode for both VCV and PCV), when evaluating the volume-controlled modes, using the set Vt as the standard, the differences between the patient and the ventilator-measured inspired Vt were 1%, 3%, 5%, and 5%, for set Vt of 50, 100, 200, and 300 mL, respectively. When evaluating the pressure-limited modes, using the ventilator mode as the standard, the differences between the patient and ventilator mode were 5% and 6% for PCV with a PIP set at 15 and 20 cm H2O, respectively. Across all volumes, the internal measurements of inspiratory Vt were closer to, and in most cases, exactly equivalent to the set volumes, than the volumes measured directly at the patient (lung analogue).
The results of the expiratory Vt from 250 in vitro measurements are included in Table 2. When evaluating the exhaled Vt, the differences were, again, statistically significant for all comparisons (patient mode versus ventilator mode for both VCV and PCV). For volume-controlled modes and using the set Vt as the standard, the differences between the patient and the ventilator measured expired Vt were 0%, 1%, 2%, and 1%, for set Vt of 50, 100, 200, and 300 mL, respectively. When evaluating the pressure-limited modes, using the ventilator mode as the standard, the differences between the patient and ventilator mode were 1% for PCV with a PIP set at either 15 and 20 cm H2O. Across all volumes, the internal measurements of expiratory Vt were closer to the set volumes than the volumes measured directly at the patient (lung analogue).
 Click to view | Table 2. Recorded Expiratory Vt |
| Discussion | ▴Top |
During mechanical ventilation, Vt is generally measured by a sensor placed at the expiratory valve or within the expiratory limb of the ventilator. Given that the internal flow meters and pneumotachometer are separate and distant from the patient, concern has been expressed regarding inaccuracies of these measurements in both the intensive care unit (ICU) and operating room (OR) setting. These concerns had previously led to the use of a pressure-controlled mode as the preferred method for intraoperative mechanical ventilation. When VCV is accurate, it is preferred over the use of PCV because PCV may have specific clinical limitations. While PCV reduces the potential for barotrauma and may be preferable in patients with acute lung injury, Vt can be variable with changes noted in the delivered Vt based on the changes in the patient’s respiratory resistance or compliance [2].
Although PCV has been the mode of choice in the pediatric ICU setting especially in smaller infants or neonates, previous work has suggested that it may be largely unreliable when the Vt is measured internally by the ventilator [6-9]. Neve et al evaluated the Vt in both pressure-controlled and volume-controlled modes using the Servo 300 ICU ventilator (Siemens-Elema, Solna, Sweden) compared to the Vt measured by a pneumotachometer placed at the connection of the circuit and the ETT [6]. Vt were overestimated by the Servo 300 in both the pressure-controlled and volume-controlled modes from 5% to 62% of the value displayed on the Servo 300 ventilator. During PCV, the maximal inspiratory pressures were underestimated by the Servo 300 ventilator by -2 to +19 cm H2O. The differences increased with increasing respiratory system impedance. Similar discrepancies were reported by Kim et al when comparing ventilator-measured Vt to those measured by a pneumotachometer in 51 intubated and mechanically ventilated pediatric patients during both pressure-limited and pressure-regulated volume controlled (PRVC) ventilation [9]. Both the ventilator-measured Vt and the Vt measured at proximal flow sensors were both significantly less than the Vt as measured by the pneumotachometer placed at the ETT. During PCV, the median Vt measured with the pneumotachometer was 9.5 mL/kg compared to 8.2 mL/kg at the ventilator or 8.1 mL/kg at the proximal flow sensor. During PRVC, the median Vt measured with the pneumotachometer was 10.2 mL/kg compared to 8.0 mL/kg at the ventilator and 8.5 mL/kg at the proximal flow sensor.
Advances in technology show promise of increasing the reliability of ICU ventilators. Yamaguchi et al evaluated the function of three different ventilators using a lung simulator set with normal resistance and compliance and those indicative of acute lung injury [10]. Three combinations of parameters were set including resistance (cm H2O/L/s) and compliance (mL/cm H2O) of 50 and 2 (group 1), 100 and 1 (group 2), and 150 and 0.5 (group 3), respectively. The lung analogue was connected to an anesthesia machine ventilator (Drager Fabius GS) and two different ICU ventilators (Servo-i Universal and Evita Infinity V500). Each ventilator was evaluated with a set Vt of 30 mL and a respiratory rate of 25 breathes/min in both the VCV and dual-controlled ventilation modes. The discrepancies between set Vt, Vt measured from the expiratory limb of the ventilator, and the Vt delivered to the patient were highest with the Fabius anesthesia machine ventilator and increased in the simulated lung injury groups. When comparing the ICU ventilators, the difference was greater in the Servo-i and increased when with simulated lung injury. The authors concluded that accurate Vt were achieved only with the infinity ICU ventilator. This was true regardless of mode of ventilation and even during simulated lung injury.
Concerns with the potential inaccuracy of mechanical ventilation have similarly been expressed when using OR anesthesia machine-based ventilators [10-12]. These changes may be impacted by type of circuit used (adult versus pediatric), as well as resistance and compliance changes of the respiratory system. As with ICU ventilators, these changes and the inaccuracies seen are dependent on the technology used. Recent advancements in intraoperative mechanical ventilation using anesthesia machine-based ventilators have suggested that the accuracy of volume ventilation has improved with advancements in technology, including machine-based compensations for breathing circuit compliance and fresh gas flow [2]. Bachiller et al demonstrated that newer generation anesthesia machines like the Aisys (GE Healthcare, Madison, WI) and Apollo (Draeger Medical, Telford, PA) which use compliance compensation software, deliver a Vt that is in a clinically acceptable range with the set Vt. However, older generation anesthesia machines which use an inspiratory flow sensor to control Vt do not.
These findings are in line with our current findings which demonstrated the accuracy of the Vt (set versus delivered) as well as the internal flow meters of the Avance CS2 Anesthesia Machine with measured Vt clinically similar to those measured at the ETT by a pneumotachometer. These findings did not change during various levels of volume and pressure-limited ventilation. However, these findings must be taken in context of the potential limitations of the study, including that this was an in vitro evaluation using a lung analogue. We did not vary the compliance and resistance of the lung analogue (respiratory system). We used a lung analogue which provided a stable, reproducible, and non-changing compliance and resistance. It may not be feasible to extrapolate our findings to in vivo situations with changing or altered compliance and resistance. Additionally, as demonstrated by previous studies, there may be significant differences based on the type of anesthesia machine used and the clinical scenario. Despite these limitations, our preliminary in vitro evaluation demonstrates that the newer models of anesthesia machines used for intraoperative mechanical ventilation provide an accurate Vt during both pressure- and volume-limited ventilation.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was not required as there were no human subjects involved. This was only an in vitro evaluation.
Author Contributions
JS: data acquisition, preparation of initial, interim, and final drafts; MT: data acquisition, preparation of drafts, and final review of manuscript; JDT: concept, writing, and review of all drafts; JRW: preparation of study protocol, IRB submission, and review of final draft; KM: study concept and design, and review of final draft.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
ETT: endotracheal tube; FiO2: fraction of inspired oxygen concentration; ICU: intensive care unit; PCV: pressure-controlled ventilation; PEEP: positive end expiratory pressure; PIP: peak inflating pressure; PRVC: pressure-regulated volume controlled; VCV: volume-controlled ventilation; Vt: tidal volume
| References | ▴Top |
- Wheeler KI, Klingenberg C, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation for preterm infants: a systematic review and meta-analysis. Neonatology. 2011;100(3):219-227.
doi pubmed - Bachiller PR, McDonough JM, Feldman JM. Do new anesthesia ventilators deliver small tidal volumes accurately during volume-controlled ventilation? Anesth Analg. 2008;106(5):1392-1400.
doi pubmed - Badgwell M, Swan J, Foster AC. Volume-controlled ventilation is made possible in infants by using compliant breathing circuits with large compression volume. Anesth Analg. 1996;82(4):719-723.
doi pubmed - Tobin MJ, Stevenson GW, Horn BJ, Chen EH, Hall SC, Cote CJ. A comparison of three modes of ventilation with the use of an adult circle system in an infant lung model. Anesth Analg. 1998;87(4):766-771.
doi pubmed - Stayer SA, Bent ST, Skjonsby BS, Frolov A, Andropoulos DB. Pressure control ventilation: three anesthesia ventilators compared using an infant lung model. Anesth Analg. 2000;91(5):1145-1150.
doi pubmed - Neve V, Leclerc F, Noizet O, Vernoux S, Leteurtre S, Forget P, Sadik A, et al. Influence of respiratory system impedance on volume and pressure delivered at the Y piece in ventilated infants. Pediatr Crit Care Med. 2003;4(4):418-425.
doi pubmed - Castle RA, Dunne CJ, Mok Q, Wade AM, Stocks J. Accuracy of displayed values of tidal volume in the pediatric intensive care unit. Crit Care Med. 2002;30(11):2566-2574.
doi pubmed - Cannon ML, Cornell J, Tripp-Hamel DS, Gentile MA, Hubble CL, Meliones JN, Cheifetz IM. Tidal volumes for ventilated infants should be determined with a pneumotachometer placed at the endotracheal tube. Am J Respir Crit Care Med. 2000;162(6):2109-2112.
doi pubmed - Kim P, Salazar A, Ross PA, Newth CJ, Khemani RG. Comparison of tidal volumes at the endotracheal tube and at the ventilator. Pediatr Crit Care Med. 2015;16(9):e324-331.
doi pubmed - Yamaguchi Y, Miyashita T, Matsuda Y, Sasaki M, Takaki S, Kim SS, Tobias JD, et al. The difference between set and delivered tidal volume: a lung simulation study. Med Devices (Auckl). 2020;13:205-211.
doi pubmed - Cote CJ, et al. Wasted ventilation measured in vitro with eight anesthetic circuits with and without inline humidification. Anesthesiology. 1983;59:443-446.
- Stevenson GW, Tobin MJ, Horn BJ, Sautel M, Chen EH, Hall SC, Cote CJ. The effect of circuit compliance on delivered ventilation with use of an adult circle system for time cycled volume controlled ventilation using an infant lung model. Paediatr Anaesth. 1998;8(2):139-144.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
