| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Original Article
Volume 13, Number 3, December 2024, pages 73-85
Feeding Behaviors Among Children With Autism Spectrum Disorder
Kajendran Visvalingama, b , Ranjini S. Sivanesoma
aChild Development and Rehabilitation Centre (CDRC), Department of Paediatrics, Hospital Tunku Azizah, Kuala Lumpur, Ministry of Health, Malaysia
bCorresponding Author: Kajendran Visvalingam, Child Development and Rehabilitation Centre (CDRC), Department of Paediatrics, Hospital Tunku Azizah, Kuala Lumpur, Malaysia
Manuscript submitted August 5, 2024, accepted November 13, 2024, published online December 2, 2024
Short title: Feeding Behaviors in Children With ASD
doi: https://doi.org/10.14740/ijcp547
| Abstract | ▴Top |
Background: Feeding difficulties are a prevalent and significant concern among children with autism spectrum disorder (ASD), affecting their nutritional status and overall health. The objectives of this study were to identify difficult feeders amongst ASD children under our follow-up, to describe the multifaceted nature of feeding difficulties in these children, to ascertain if these findings were similar to those in other populations and finally to identify children who are underweight and obese whilst looking at the correlation to feeding struggles. Children with ASD often exhibit a range of feeding difficulties, including food selectivity, mealtime behaviors, and sensory sensitivities. These challenges are commonly associated with the fundamental characteristics of ASD, including repetitive behaviors and sensory processing issues. These traits can result in strong preferences for food textures, colors, or types, as well as aversions to others.
Methods: This is a cross-sectional study of children diagnosed with ASD between 1 and 7 years on follow-up at the Child Development and Rehabilitation Center (CDRC), Hospital Tunku Azizah, Kuala Lumpur between October 2022, and August 2023. The Brief Autism Mealtime Behavior Inventory (BAMBI) and Montreal Children’s Hospital Feeding Scale (MCH-FS) were used to assess children’s mealtime behaviors. BAMBI contains 18 items which are scored by parents on a one to five-point Likert scale. It entails domains of limited food variety (eight items), food refusal (five items) and features of autism (five items). MCH-FS contains 14 items which are scored by parents on a one to seven-point Likert scale. It entails domains of parental concern (three items), family reaction (two items), compensatory strategy (three items), appetite (two items), mealtime behavior (two items), oral sensory (two items) and oral motor (two items).
Results: The sample consisted of 70.7% male and 29.3% female children from numerous ethnic backgrounds. Most of the children were ASD level 2 and had received the diagnosis of ASD at an average of 40 months. Among the 341 children, 72.7% (248) parents reported feeding difficulties on the BAMBI and 44.6% (152) on MCH-FS. The BAMBI mean total score of 41.46 was comparable to similarly studied Asian populations but was significantly lower compared to Western populations. This suggests cultural similarities of feeding challenges amongst Asian ASD population. Understanding cultural differences in caregivers’ beliefs and feeding styles can improve the effectiveness of feeding strategies. We also found that higher levels of repetitive restrictive behavior (RRB) were associated with a greater likelihood of feeding difficulties. These findings imply that interventions addressing RRB should be considered for their possible benefits on tackling feeding difficulties. Restricted food intake can lead to nutritional insufficiency if the types and variety of the food remain limited. Interestingly, 25.2% (86) of the studied population were underweight (body mass index (BMI) < fifth percentile) whereas 9.1% (31) were categorized as obese (BMI > 95th percentile). The median BAMBI score was higher in the overweight and obese group, indicating more severe feeding behaviors. This statistically significant difference indicates BMI may have an influence on the overall outcomes measured by BAMBI.
Conclusions: Problematic feeding behavior is a major problem amongst ASD children, and limited food repertoire remains the highest reported domain. BAMBI and MCH-FS questionnaires provide excellent overview to identify feeding difficulties amongst children with ASD. Identifying causative domains of the behavior enables clinicians to establish focused interventions in managing difficult feeders.
Keywords: Autism spectrum disorder; Feeding behaviors; Children; BAMBI; MCH-FS; Pediatric nutrition; Behavioral analysis
| Introduction | ▴Top |
Clinicians require access to a valid and reliable instrument that can quickly verify parental concerns about their child’s feeding problems which may go unnoticed [1]. Approximately 25% of children face feeding-related issues during their early years. However, this number can be as high as 80% in children with developmental difficulties including autism spectrum disorder (ASD) [2-4]. Mealtime behavior or feeding problems are not routinely addressed unless a child exhibits failure to thrive, which could be the driving reason for lack of research on feeding issues amongst children with ASD [2, 5]. ASD is a neurodevelopmental disorder characterized by impairments in communication, behavior, and social functioning, beginning in childhood. We lack local epidemiological data on prevalence of ASD in Malaysia. However, according to a Malaysian Ministry of Health feasibility study on the use of the Modified Checklist for Autism in Toddlers (M-CHAT) among children aged 18 - 36 months, the prevalence of ASD was around 1.6 per 1,000. This is much lower than the rates reported in recent global prevalence studies of ASD and is probably an underestimate [6, 7]. The trend of ASD has increased over the years worldwide. Autism prevalence has been showing an increasing trend over the past few decades. The Center of Disease Control and Prevention established the Autism and Developmental Disabilities Monitoring (ADDM) Network in 2000 to track ASD prevalence in the United States. The overall ASD prevalence per 1,000 children aged 8 years was 27.6 (one in 36), and the overall male-to-female prevalence ratio was 3.8 [8].
There are a diverse range of feeding issues such as picky eating, restricted food repertoire and food refusal. Systematic review on feeding problems in ASD provided robust evidence of significant feeding problems in this population [9]. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) definition of clinical feeding problems includes pica, rumination disorder and avoidant/restrictive food intake disorder (ARFID), which requires persistent failure to meet nutritional or energy needs together with psychosocial functioning. Whilst some feeding problems fit within these criteria, the broader spectrum of feeding difficulties that are experienced by children and their parents can vary widely in type and cause. Notably it includes poor appetite, sensory processing difficulties, neophobia, poor oral-motor feeding skills, problematic mealtime behavior and difficult feeding interaction with parents. In addition to potential physical health impacts, feeding difficulties also encompasses parental stress and sense of competence, emotional wellbeing, and parent-child interaction [10].
Another imperative nutritional deficiency associated with ASD is obesity. In a local study, it was found that the prevalence of obesity and overweight is high among children and adolescents with ASD. One of the risk factors identified for this includes the high likelihood of food selectivity [6]. Generally, the terms “feeding difficulties” and “feeding problem” are commonly used to specifically denote problems in early childhood, which do not necessarily lead to significant nutritional deficiency. Many children with ASD do not perceive sensory inputs in the same manner as their typically developing peers of same age, particularly tactile, olfactory, and visual/auditory information [2]. Selection of food type (temperature, texture, brand, and color), presentation, placement (type of cutlery, brand of cutlery, organization food on plate) and the environment in which the food is served can affect the feeding in ASD children [11]. Seiverling et al reported that children with ASD showed more food selectivity by texture and type compared to a non-ASD child with language delay [12].
Feeding behavior is another entity of feeding difficulties in children with ASD. Some of the reported behavior includes walking away from the table, whining and yelling, throwing and dumping, and also tantrums during eating [9]. Children with ASD are also noted to have rituals around food which can often lead to much anxiety during mealtimes. It is also postulated that the mealtime behavior is directly linked with the ASD characteristics. Many studies have shown the association between more severe parent-reported feeding difficulties and ASD sensory sensitivities. However, it remains to be determined if ASD symptoms severity is associated with mealtime behavior. Patton found that children with more severe ASD were less likely to try new food [13]. However, there was no significance found between ASD severity and disruptive mealtime behavior.
Clinicians should be on high alert to detect feeding problems in early childhood to ascertain timely referral for further management. There are various screening instruments that can be used to detect feeding difficulties amongst children [14]. Existing questionnaires include the Behavioral Pediatrics Feeding Assessment Scale (BPFAS), the Children’s Eating Behavior Questionnaire (CEBQ) and the Mealtime Behavior Questionnaire (MBQ).
At present, there is very little research on this topic amongst our local population. This study aims to identify the prevalence and describe the nature of feeding-related issues amongst children with ASD in our population. This also addresses the need for early identification and intervention when feeding difficulties are detected in children with ASD.
| Materials and Methods | ▴Top |
Study participants
Parents of children aged 1 to 6 years 11 months with ASD were invited to participate in the study. These patients were under the follow-up of Child Development Clinic (CDC), Hospital Tunku Azizah, a tertiary Women and Children Hospital in Kuala Lumpur, Malaysia. All children followed up at the CDC are evaluated by a developmental pediatrician, who makes a clinical diagnosis of ASD based on the DSM-5. Certain instances where further evaluation was needed, the Autism Diagnostic Observation Schedule (ADOS-2) was conducted by trained professionals based at CDC.
Study design
This is a cross-sectional study. After signing the consent form, the parent/caregiver were given two sets of questionnaires. Brief Autism Mealtime Behavior Inventory (BAMBI) [15] and Montreal Children’s Hospital Feeding Scale (MCH-FS) [1] in English language were used with permission from the original authors. The BAMBI is an 18-item questionnaire with Likert scales. Whereas the MCH-FS is a 14-item questionnaire with Likert scales. Both these questionnaires are parent-reported, and investigators assisted only when necessary. Patient’s weight (kg) and height (cm) were obtained using a standard weighing scale and a stadiometer respectively. The body mass index (BMI) was measured manually, and these parameters were transcribed into the Center of Disease Control and Prevention charts based on age and gender. As defined by the Center of Disease Control and Prevention, underweight is defined when the BMI is < fifth percentile, overweight when BMI is between 85th and 95th percentile, and obesity when the BMI > 95th percentile for the appropriate age and gender. Respondent’s weight, height and BMI were also obtained in similar fashion. Electronic medical records of these children were screened to obtain further demographic data including comorbidities, age of diagnosis, and family history. This study was conducted with ethical principles outlined in the Declaration of Helsinki and Malaysian Good Clinical Practice guidelines. The ethical approval was obtained from the Malaysian Medical Research Ethics Committee. This study was registered with the National Malaysian Medical Registry under NMRR ID: 22-01852-ANP (IIR).
Recruitment
The study was conducted from October 2022 to December 2023. Parents or caregivers of children who met the inclusion criteria were approached by the investigator either during their initial presentation or follow-up at the CDC. Children with existing organic feeding disorders such as gastroesophageal reflux disease (GERD) and other chronic medical conditions significantly affecting feeding were excluded from the study. Additionally, children taking medications known to suppress appetite, such as methylphenidate, were also excluded. Parents or caregivers who had difficulty with the questionnaire due to limited English proficiency were assisted in completing it but were ultimately excluded from the final study sample. The severity of ASD in the children was classified according to the DSM-5 criteria, ranging from level 1 indicating “requiring support”, level 2 indicating “requiring substantial support” and level 3 indicating “requiring very substantial support”, respectively. These classifications were applied to both domains of social communication and interaction, as well as restrictive and repetitive behaviors.
Assessment tools
Two parent self-administered questionnaires were used.
BAMBI
BAMBI was developed by Dr. Carol Taylor Lukens as the first standardized informant report measure to capture mealtime and feeding behavior in a detailed manner in children with ASD [15]. The BAMBI was developed in response to the limitations of other measures not being sensitive to the behaviors found in children with ASD [16]. The 18-item measures employ a Likert scale for reporting the frequency of behaviors (1 = never/rarely to 5 = at almost every meal) [17]. It was originally developed with a sample of 108 children, out of which 68 were with ASD, with its brevity as its strength in comparison to other measures: 1) limited variety; 2) food refusal; 3) features of autism.
The first criterion, limited variety, refers to the limited food accepted by the child. The second, food refusal, points to the behavior the child does when refusing food. The third is features of autism which includes maladaptive behavior and limited flexibility with feeding and mealtime. The BAMBI questionnaire was previously translated and used in a local study which looked at the prevalence of obesity amongst autistic children [6]. However, in this study we used the original version in English.
MCH-FS
MCH-FS was developed by Ramsay et al based on the observation that clinical and non-clinical groups show similar behavior around feeding [1]. However, children with feeding difficulties show these behaviors at a higher frequency. The items in the scale were created in accordance with the biopsychosocial model of feeding problems. This 14-item scale measure seven main constructs: 1) parental concern (M1, M2 and M12); 2) family reactions (M13 and M14); 3) compensatory strategies (M5, M9 and M10); 4) appetite (M3 and M4); 5) mealtime behavior (M6 and M8); 6) oral sensory behavior (M7 and M8); 7) oral motor behavior (M8 and M11).
This valid and reliable test has also been translated in French, Dutch and Thai [1, 9, 18]. Parents report on each item using a Likert scale 1 - 7. MCH-FS was investigated in large normative population samples and in various clinical groups, such as Down syndrome, premature children and children with cleft palates. There was some overlap between both questionnaires with regards to sensory motor problems, food refusal, negative mealtime behavior and selectivity. The issue of MCH-FS being a suitable tool to assess the ASD population was addressed in the study of van Dijk et al [9], which showed that MCH-FS scales can be used in populations that include children with ASD. This questionnaire is available in English [9].
Both these parents-reported questionnaires were used. The BAMBI is specifically designed to assess mealtime behaviors in children with autism, focusing on issues like food selectivity, sensory sensitivities, and rigid eating routines. In contrast, the MCH-FS is a broader tool used to assess general feeding problems in children, regardless of whether they have autism, and includes issues like food refusal and mealtime disruptions. BAMBI targets autism-related feeding challenges, while MCH-FS evaluates feeding difficulties across various conditions.
Using both the questionnaires simultaneously can provide a more comprehensive understanding of a child’s mealtime behaviors, especially in children with autism. It provides a holistic assessment identifying both autism-related behaviors (e.g., sensory sensitivities, rigid routines) and other feeding difficulties (e.g., food refusal, mealtime disruptions), giving a fuller picture of the child’s feeding patterns. By using both assessments, clinicians can address the full spectrum of a child’s feeding issues, considering both autism-related factors and general feeding problems. This can lead to a more tailored and effective treatment plan, addressing both sensory needs and behavioral feeding challenges. Finally, if a child has both autism-specific and more general feeding difficulties, using both tools allows for monitoring progress in different aspects of feeding over time, ensuring that interventions are addressing all relevant factors.
If a child’s assessment indicates notable feeding challenges, the parent/caregiver will be notified. With their consent, appropriate referrals will be made to an occupational therapist to address behavioral and sensory processing issues. If there are concerns about the child’s weight, height, or BMI measurements, steps will be taken to discuss and address these concerns, including referral to a dietitian if necessary. Significant scores on assessment tools such as the BAMBI are considered to be 34 or higher. Similarly, for the MCH-FS, a raw score greater than 45 (T score above 60) indicates significant issues [1].
Data analysis
Data analysis was performed using Statistical Package for the Social Sciences (SPSS) Version 26 with statistical significance set at P< 0.05. Quantitative variables were expressed as means and standard deviation. While the qualitative variables were expressed as frequency and percentages. In the analysis, Chi-square correlation analysis were used for all tests. The level of significance was set at 0.05.
| Results | ▴Top |
Background characteristics of the children
A grand total of 341 replies were gathered from parents and guardians. All of the children had a confirmed diagnosis of ASD, with the average age at which they were diagnosed being 39 months. Approximately 106 children, accounting for 31% of the total, were aged between 6 years and 6 years 11 months, whereas 241 children, making up 70.1% of the total, were male. Out of the total of 341 youngsters, who were from various ethnic backgrounds, 229 of them, accounting for 67% of the group, were of Malay origin, which reflects the ethnic distribution in Malaysia. In every age group, a greater proportion of them are either underweight or have a BMI that is within the normal range. The average weight of the youngsters was 17.8 kg, and their height was 108 cm. It was further determined that these children with ASD were classified into levels 1 through 3 for both social communication interaction (SCI) and repetitive, restricting behavior (RRB), which indicated the level of support the child required. One of the common comorbidities seen in this population was global developmental delay (GDD) for those children below 5 years of age. A significant part of the parents who responded were aged between 31 and 40 years and had obtained a bachelor’s degree or lower level of education. Approximately 37% of the households belonged to the income bracket of Malaysian ringgit (RM) 5,000 - 10,000 per month. In addition to parents, other relatives that participated as respondents included grandparents and aunts. The two caretakers consisted of a nanny and a shadow aid, respectively. The demographic characteristics are illustrated in Table 1.
 Click to view | Table 1. Demographic and Socioeconomic Characteristics of the Sample (N = 341) |
Descriptions of feeding difficulties using the BAMBI and MCH-FS
Figure 1 represents the distribution of feeding difficulty among patients assessed using the BAMBI questionnaire. Of the total 341 patients evaluated, a substantial majority (72.7%, n = 248) were classified as having a feeding difficulty as they scored 34 and above in the BAMBI questionnaire. In contrast, 27.3% (n = 93) of the parents reported that children did not have any feeding difficulty.
 Click for large image | Figure 1. Feeding difficulty classification among patients (BAMBI, n = 341). BAMBI: Brief Autism Mealtime Behavior Inventory. |
We then looked into this study and compared the findings of similar studies using BAMBI questionnaire in other populations, as depicted in Table 2 [11, 15, 19]. Kang et al compared feeding habits on Asian Singaporeans whilst Gray et al studied Chinese Americans [11, 19]. Comparisons were also done by authors of BAMBI (Lukens et al [15]).
 Click to view | Table 2. BAMBI Results With Comparison to Populations of Different Ethnicity |
The total scores and subscale scores (limited variety, food refusal, features of autism) from this study were compared to scores from similar studies on different populations [11, 15, 19]. This study’s mean total score (41.46) was similar to that from the study of Kang et al [11] (40.8), and lower than both from Gray et al [19] (43.59), and Lukens et al [15] for ASD (49.05), but higher than their typically developing group (32.50). When we looked into each construct, this study reported a higher mean score (22.36) for limited variety, compared to that from the study of Kang et al [11] (20.7), and Gray et al [19] (23.17), but lower than that from the study of Lukens et al [15] for ASD (27.61). Interestingly, food refusal mean scores in this study (9.49) was lower than that from the study of Kang et al [11] (11.2), and Lukens et al [15] for ASD (10.36), but higher than that from Gray et al [19] (9.33) and Lukens et al [15] for typical developing (6.93). This study had a mean score (9.61) in the construct of autism features, which was higher than that from the study of Kang et al [11] (8.8), but lower than Gray et al [19] (11.09), and Lukens et al [15] for ASD (11.07), but higher than their typically developing group (7.75).
The second questionnaire used in this study was MCH-FS. Figure 2 categorizes the feeding difficulty among a cohort of 341 patients assessed using the MCH-FS criteria. Interestingly, it was demonstrated that the majority of the patients (55.4%, n = 189) were classified as not having feeding difficulty, as they scored 45 and below for the raw score (T-score: 60 and below). However, a significant portion (44.6%, n = 152) was categorized as having feeding difficulty, with raw score of more than 45 (T-score: more than 60). This could be further divided into mild feeding difficulty with raw score of 46 - 52 (T-score: 61 - 65), moderate feeding difficulty with raw score of 53 - 58 (T-score: 66 - 70), and finally severe feeding difficulty with raw score of 59 and above (T-score: 71 and above).
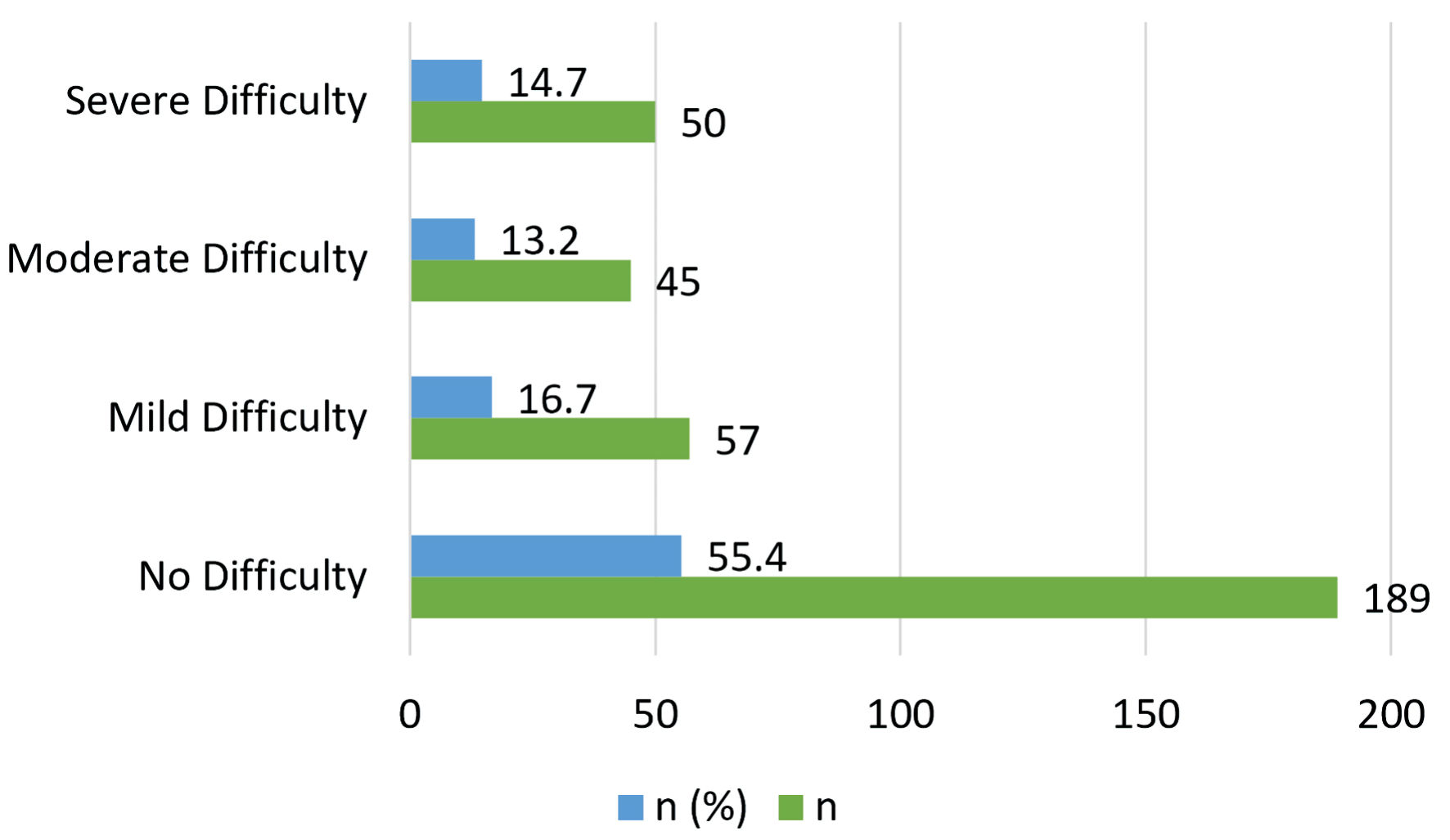 Click for large image | Figure 2. Feeding difficulty classification among patients (MCH-FS, n = 341). MCH-FS: Montreal Children’s Hospital Feeding Scale. |
Figure 2 presents the distribution of feeding difficulty levels among the children based on their MCH scores. Each category includes the count and percentage of the total sample size (n = 341).
Like the BAMBI, the MCH-FS was also used in other similar studies looking into feeding difficulties amongst children worldwide. As shown on Table 3, we compared our results with those of Benjasuwantep et al [18], who studied feeding difficulties in Thai children, Ramsay et al, the authors of MCH-FS, who primarily focused on Canadian children [1], and van Dijk et al, who studied the Dutch children [9].
 Click to view | Table 3. Comparison of MCH-FS Questionnaire Item Scores Across Different Populations |
We compared the scores of the MCH-FS obtained from our population with other populations who used the similar questionnaire when addressing feeding difficulties in children. The mean individual item scores of this study in the MCH-FS compared well with those of the clinical samples from the Thai, Dutch and Canadian populations, as demonstrated in Table 3. The range of the mean scores of the 14 items was almost the same in the three versions. Detailed item-specific observation, as shown in item M1 which assessed difficult mealtimes, revealed that the Canadian clinical sample from Ramsay et al, reported the highest difficulty (5.30), followed by the Dutch clinical sample from van Dijk et al (3.99), this study (3.20), and the Thai study by Benjasuwantep et al (2.65) [1, 9, 18]. Item M2 showed the highest worries were reported in the Canadian clinical sample (5.49, Ramsay et al), with the lowest in the Thai study (2.94) by Benjasuwantep et al [1, 9, 18]. The Canadian clinical sample (Ramsay et al) reported significant issues with poor appetite M3 (4.60), while the Thai study (Benjasuwantep et al) showed the least (2.68) [1, 9, 18]. When comparing M4 onset of food refusal, Canadian (5.42, Ramsay et al) and Dutch clinical samples (4.90, van Dijk et al) reported higher scores, indicating severe issues compared to this study (4.02) and the Thai study (2.75, Benjasuwantep et al) [1, 9, 18]. Similarly, M11 poor chewing was observed in the Dutch (3.31, van Dijk et al) and Canadian clinical samples (3.32, Ramsay et al), which were higher than that from this study (2.43) and the Thai study (1.49, Benjasuwantep et al) [1, 9, 18]. Both the Canadian and Dutch clinical samples showed higher mean scores across most items, reflecting greater feeding difficulties in children with ASD. The general population had lower mean scores, indicating fewer feeding difficulties, while clinical samples show elevated issues [18]. The mean scores in this study were generally higher than those in the Thai study (Benjasuwantep et al) but lower than those in the clinical ASD samples, suggesting moderate feeding difficulties.
Relationship between autism levels and feeding difficulties
Factors associated with feeding disturbances in children with ASD are considered to be multifactorial (sensory, behavioral, psychological, communicative, or familial factors). Research has indicated that feeding difficulties may be both a cause and a consequence of ASD, with much variability reported in the literature. Some studies have indicated that the conceptualization of feeding problems in children with ASD may manifest as anatomical, metabolic, gastrointestinal, motor or sensory difficulties, resulting in an increased risk for gastrointestinal problems. However, there have also been studies that have suggested that abnormal eating patterns may trigger gastrointestinal difficulties and alter the gut microbiome in children with ASD [20]. Therefore, it is unclear whether children with ASD have a predisposition to gastrointestinal problems, or if food preferences may in fact trigger gastrointestinal issues. Despite our understanding of ASD and feeding difficulties, there is currently no defined etiology for these noted challenges. Feeding difficulties are heterogeneous in nature and may be further complicated by other medical problems, as well as the child’s food choices and general mealtime behaviors.
We looked into the relationship between the level of support needed for both the diagnostic criteria for ASD, SCI, and RRB, and the feeding difficulties described in our population, as shown in Tables 4 - 7.
 Click to view | Table 4. Cross-Tabulation of SCI With Feeding Difficulty Classification (BAMBI) |
 Click to view | Table 5. Cross-Tabulation of RRB With Feeding Difficulty Classification (BAMBI) |
 Click to view | Table 6. Cross-Tabulation of SCI With Feeding Difficulty Classification (MCH-FS) |
 Click to view | Table 7. Cross-Tabulation of RRB With Feeding Difficulty Classification (MCH-FS) |
The relationship between SCI levels and feeding difficulty classification (BAMBI) was not statistically significant (P > 0.05 in all tests). The proportions within each SCI category were similar across the different levels of feeding difficulty, indicating no strong association between SCI and feeding difficulty based on the BAMBI classification. However, there was a statistically significant association between RRB levels and feeding difficulty classification (BAMBI) (P < 0.05). Higher levels of RRB were associated with a greater likelihood of having feeding difficulties. This suggests that interventions targeting RRB might need to consider the potential impact on feeding difficulties.
There was no statistically significant association between SCI levels and feeding difficulty classification (MCH- FS) (P > 0.05). The distribution of SCI did not appear to influence the classification of feeding difficulty as defined by the MCH-FS criteria. Interestingly once again the Chi-square results suggested that there was a statistically significant association between RRB levels and feeding difficulties as categorized in the MCH-FS. For the ASD children with RRB level 1, the majority of children (62.5%) did not have feeding difficulties, which could imply that lower levels of restrictive and repetitive behaviors are less associated with severe feeding issues. However, children with RRB level 2 displayed nearly equal distribution between having and not having feeding difficulties, indicating that as RRB severity increased, so did the likelihood of encountering feeding difficulties. Although the sample size was small (n = 4), a significant percentage (75%) had feeding difficulties, strongly suggesting that higher RRB levels correlated with more severe feeding challenges.
Feeding difficulties in ASD and its impact on the weight
As depicted in Table 8, the median BAMBI total score was higher in the overweight and obese group (43.00), compared to the normal and underweight group (40.00). The overweight and obese group showed a slightly higher overall score, which might suggest a greater prevalence or intensity of the behaviors assessed by BAMBI in this group. The difference was statistically significant (P = 0.034), indicating that BMI may have an influence on the overall outcomes measured by BAMBI. When looking in depth for each BAMBI domain, the score for limited variety was also higher in the overweight and obese group (25.50), compared to the normal and underweight group (22.00), with statistical significance difference (P = 0.003), suggesting that children who were overweight or obese may have a more limited variety of food preferences or acceptances. When looking at food refusal, there was a slight difference in median scores between the groups, but it was not statistically significant (P = 0.844). Both groups exhibited similar levels of food refusal, indicating that BMI may not play a significant role in this particular behavior. Similarly, no statistical significance (P = 0.343) was noted despite slightly higher scores in the overweight and obese group (10.00) compared to the normal and underweight group (9.00). The similarity in scores suggests that autism-related features assessed may not vary significantly with BMI.
 Click to view | Table 8. Comparison Between Normal and Underweight ASD Children With Overweight and Obese ASD Children Based on Domains of BAMBI and MCH-FS |
Interestingly, the MCH-FS total score was higher in the normal and underweight group (43.00) than in the overweight and obese group (38.00), but this difference was not statistically significant (P = 0.072). This suggests that while there may be a trend towards higher behavioral concerns in the normal and underweight group, it is not significantly affected by BMI. Parental concern domain showed significantly higher concerns in the normal and underweight group (P = 0.007). Parents of children in the normal and underweight group may experience more concerns, potentially reflecting sensitivity to the children’s eating habits or health. Another domain which showed statistical significance was compensatory strategy (P = 0.018), with the normal and underweight group scoring higher. This might indicate more strategies being employed to manage or compensate for eating issues among the normal and underweight group. Domains like appetite, mealtime behavior, oral sensory, and oral motor showed no significant differences in scores between the groups. These behaviors appear consistent across BMI categories, suggesting that these specific mealtime behaviors and sensory responses are less influenced by weight status.
The findings indicate that certain behavioral aspects related to eating and mealtimes are influenced by BMI, particularly in terms of parental concern and compensatory strategies. These differences highlight the potential impact of BMI on children’s eating behaviors and parental perceptions, warranting further investigation to understand the underlying causes and implications for interventions.
Interpretation of correlation analysis between BAMBI and MCH total scores
The correlation analysis between the BAMBI total score and the MCH-FS total score was conducted using both Pearson correlation coefficients. The Pearson correlation coefficient was 0.696, with a statistically significant P value of 0.000 (two-tailed). The sample size was 341 participants.
The Pearson correlation coefficient was 0.696, indicating a strong positive relationship between the BAMBI total score and the MCH total score (P < 0.001). This suggests that higher scores on the BAMBI, which indicate more severe mealtime behaviors, are associated with higher scores on the MCH-FS, indicating more severe feeding difficulties. Table 9 shows the agreement between the BAMBI and MCH classifications of feeding difficulty.
 Click to view | Table 9. Crosstabulation of Feeding Difficulty Classification on BAMBI and MCH-FS |
In the BAMBI questionnaire, amongst those who had raw score of < 34, 83 children (43.9% of those classified as “not having difficulty” group as per MCH-FS) were categorized as “less than 34” by BAMBI; 10 children (6.6% of the “having difficulty” group as per MCH-FS) were classified as “less than 34” by BAMBI; in total, 93 children (27.3% of the total sample) scored “less than 34” (BAMBI). For those who had raw score of 34 and above (BAMBI): 106 children (56.1% of those classified as “not having difficulty” group as per MCH-FS) were classified as “34 and above” by BAMBI; 142 children (93.4% of those classified as “having difficulty” group as per MCH-FS) were classified as “34 and above” by BAMBI. In total, 248 children (72.7% of the total sample) scored 34 and above (BAMBI). Overall, 189 children (55.4% of the total sample) were classified as “not having difficulty” (MCH-FS); 152 children (44.6% of the total sample) were classified as “having difficulty” (MCH-FS); and the total sample consisted of 341 children (100.0%).
Cohen’s Kappa value was 0.352, which indicates fair agreement between the BAMBI and MCH-FS classifications of feeding difficulty. This value was significant at the P < 0.01 level (P = 0.000), suggesting that the observed agreement is statistically significant and not due to chance. According to general guidelines, a Kappa value of 0.352 falls into the “fair” agreement category.
| Discussion | ▴Top |
The proportion of feeding challenges among children with ASD is 0.7273 with 95% confidence interval from 0.6776 to 0.7719. Despite this clinical concern, the number of children who are underweight or overweight/obese is relatively low. Research indicates that while feeding difficulties are five times more common in neurodiverse children compared to neurotypical children, these issues often receive less attention than other clinical concerns within this population [21]. One possible explanation is that many of these children maintain normal or satisfactory growth despite their feeding issues, often due to compensatory measures such as supplementing meals with formula feeds, implemented by parents. However, concerns about potential nutritional deficiencies are often overlooked unless a detailed dietary history is obtained and addressed. In addition, the regularity of weight and height measurements for these children may not be consistently documented throughout time, despite being collected on every clinic visit. Therefore, although only 25% of children may weigh less than the third percentile for their age, this figure could underestimate the prevalence of growth delay, particularly given that feeding difficulties may persist over extended periods in older children.
A recurring issue among parents of children with feeding difficulties is the lack of variety and nutritional balance in their children’s diets. Many children with ASD have a strong preference for processed and carbohydrate-dense foods, often avoiding healthier options such as fruits, vegetables, and proteins. This dietary pattern can result in nutritional deficiencies, leaving these children susceptible to various health problems [17]. Research by Bandini et al highlighted that children with ASD exhibited higher levels of food refusal and possessed a more limited food repertoire compared to their typically developing peers [22]. This tendency for food refusal was present in both groups, but it was significantly more pronounced in children with ASD. Contrary to the common belief that dietary pickiness decreases with age, their cross-sectional study revealed that typically developing children maintained similar levels of food refusal and variety across different ages. In contrast, children with ASD showed a slight decrease in food refusal as they grew older, but their food repertoire did not expand. This study aligns with these findings, with parents frequently expressing concerns about their children’s limited food variety. This restricted diet, characterized by extreme picky eating and selective food preferences, poses significant nutritional challenges. Children with highly selective eating habits face two primary risks related to nutritional deficiencies. On one end of the spectrum, some children remain underweight due to the lack of essential nutrients, while on the other end, some children become obese because their diet lacks a balanced variety of foods. This problem is further illustrated by two studies [23, 24], which reported cases of scurvy among children with neurodevelopmental concerns. Scurvy, a condition caused by a severe deficiency of vitamin C, was observed in children with ASD as well as previously healthy children, emphasizing that a restricted diet is a significant common thread in these cases. These studies underscore the critical need for addressing dietary variety and nutritional balance in children with ASD to prevent such severe health outcomes.
This study used two validated questionnaires to detect the feeding difficulties in children with ASD. As shown in Figure 1, we could identify feeding difficulties amongst 72.7% children as reported by parents. However, we noted a discrepancy of the results when MCH-FS was used, as shown in Figure 2, which showed a lower 44.6% children with feeding difficulty. The authors suggested that parents could have been non-committal when reporting their children’s behavior, possibly due to the large scale (1 - 7). This is in contrast to the BAMBI questionnaire, which has a smaller scale (1 - 5). To analyze the potential impact of scale length on responses to the MCH-FS and BAMBI, we compared the distributions of responses and tested if there was a central tendency bias. Descriptive statistics were used to compare the means and standard deviations of both questionnaires. We then examined the frequency distributions of responses for each item on both scales to identify if there was a tendency to choose the middle option. Median and mode analysis and central tendency bias test were conducted. We could conclude that a larger scale (1 - 7 for MCH-FS) might lead to central tendency bias, where respondents choose the middle option more frequently. The analysis can quantify this effect and compare it with a smaller scale (1 - 5 for BAMBI). This would explain the lower feeding difficulties reported when using the MCH-FS questionnaire.
The results obtained from the BAMBI questionnaire were comparable to those from similar studies conducted in different populations. This study showed a total score of 41.46, which is similar to the study of Kang et al [11], who used the same questionnaire in Singaporean Asian population. The results were slightly different to those from Lukens et al [15] and Gray et al [19]. This suggests potential cultural similarities in the populations. If we break down the constructs of the questionnaire, the domain related to limited variety is the main concern reported by parents. The definition of food selectivity was categorized into three domains: food refusal, limited food repertoire, and high single frequency food intake. If a child experienced any one of the three domains, they were food selective. Various studies have shown that children with autism exhibit greater selectivity when compared with typically developing children. In children who have ASD, selective eating can be more extreme and may extend beyond early childhood. Clinicians and parents describe children with ASD and accompanying food selectivity as only eating foods of a particular texture, color or flavor of a particular plate and/or with certain utensils. This food selectivity poses a significant concern for parents because it has been associated with inadequate nutrition intake and is often accompanied by disruptive mealtime behavior [25].
One of the secondary objectives of the study was to determine if the severity of autism is associated to the severity of feeding difficulties. Severity of autism is graded based on the level of support the child needs, which is between 1 and 3. When we looked at the SCI component, there was no statistical significance to suggest increasing feeding difficulty. Interestingly, when authors compared the RRB component, there was statistical significance. This would suggest that the increasing level of support of RRB would be associated with increasing feeding difficulty. Numerous factors can contribute to feeding difficulties, including restrictive behavior and limited food repertoire. A child with level 3 RRB ASD may struggle with sensory issues, which cause them to avoid certain textures, flavors, colors and taste, inadvertently limiting their variety. Margari et al also reported a link between narrow diet and repetitive/ ritualistic behaviors, suggesting eating difficulty can be extensions of the core symptoms of ASD [4]. This then subsequently can lead to growth failures including failure to thrive or even obesity.
Restrictive and repetitive activity can significantly influence mealtime behaviors, perhaps resulting in selective eating. Children diagnosed with autism spectrum condition may exhibit a strong preference for specific foods based on their appearance, size, and the type of utensils used during mealtimes. Moreover, the ritualistic and repetitive behaviors of ASD, as well as the strong desire for consistency, “insistence for sameness”, and behavioral inflexibility are likely to contribute to rigid mealtime routines. In the context of feeding and mealtimes, repetitive behaviors and ritualistic routines might include demands for specific utensils and dishware, order of food presentation, insistence on food not touching, and sitting in specific places at the table. It is postulated that these challenging or problem eating behaviors can severely compromise nutrient intake. Recognizing and accommodating a child’s preference for consistency and rigidity may yield better results than a direct approach that disregards the child’s rituals, which could potentially increase anxiety [25].
The negative consequences of feeding difficulty have an impact not just on the affected child but also on the parents/caregiver. Parents of children with ASD have been demonstrated to experience increased parental stress compared to parents of typically developing children and children with other disabilities including other developmental disorders [26, 27]. In addition to the personal and interpersonal toll that parenting stress can affect both mothers and fathers [28], high levels of parenting stress can result in parent-child conflict and inter spouse stress [17, 29]. This results in a decreased ability to implement important interventions that could benefit their child. Parents must not only address potential dietary deficiencies and health risks, but also handle their child’s difficult behaviors during mealtimes, which can cause stress and impair the overall functioning and routines of the family [30]. Children with ASD not only display pickiness about what they will eat but can also have intense reactions to the texture, smell, and presentation [10]. Aversive behaviors related to feeding exhibited by many children with ASD make mealtimes stressful for multiple family members, sometimes require separate meals or mealtimes for different family members, and often result in an inability to eat outside the home with the child with ASD.
Although this was something we did not report in this study, it was apparent that parents would express their concerns regarding feeding problems when managing their ASD child.
Limitations and future directions
The population sample used in this study was not selected randomly. Instead, a convenient selection strategy was employed, where parents of children with ASD who visited the CDC clinic were offered the opportunity to participate. The use of parental report questionnaires may have also created less accuracy in results. Parents may also have limited the food variety owing to pre-existing rigidity in the child’s behavior just to ensure the child readily accepts the food during the mealtime [3]. Parenting styles and parental feeding practices were not explored. There could be varying feeding practices that parents adapt to, which are culturally inclined. One future direction for research in this area would also include direct observation of parent-child mealtime interactions to identify a coding system to track and capture both parent and child behavior, which could be modeled after procedural integrity procedures outlined in single-subject research [31].
Conclusions
Problematic feeding behavior is a major challenge amongst children with ASD. This study reports a prevalence of 73% amongst the studied population. Amongst the feeding difficulties described, limited food variety remains the most significant concern of feeding difficulty, and limited food repertoire remains the highest reported domain. The findings of the study were comparable to those obtained from other Asian populations that were studied in a similar manner. The levels of RRB are correlated with a greater risk of having feeding difficulties, and there is a statistically significant association between the two. The higher the levels of RRB, the greater the likelihood of it being associated with feeding difficulties. Given this, it appears that interventions aimed at tackling RRB should be taken into consideration when addressing these problematic feeders. In conclusion, both the BAMBI and the MCH-FS questionnaires offer a comprehensive overview that is useful for identifying eating difficulties in children who have ASD. It is possible for physicians to design targeted interventions for the management of problematic feeders if they are able to identify the domains of behavior that are responsible.
Acknowledgments
The authors would like to thank the parents/carers of children with ASD and acknowledge their participation during the study. This work would not have been possible without their contribution. We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Financial Disclosure
This research was not funded.
Conflict of Interest
This research was done by Dr. Kajendran Visvalingam during his Fellowship in Developmental Pediatrics (2022 - 2023) at the Child Development and Rehabilitation Center, Hospital Tunku Azizah, Kuala Lumpur, Malaysia.
Informed Consent
Written informed consent was obtained from the parent/carer before enrolment into the study.
Author Contributions
KV was responsible for protocol development, patient enrolment, data collection, storage and analysis, preparation of the initial and subsequent drafts, figure and table design, and review of the final draft. RS contributed to conceptualization, protocol development, study design, recruitment of the patients, supervision and manuscript review.
Data Availability
The data that support the findings of the study are available upon request from the corresponding author.
Abbreviations
ADOS: Autism Diagnostic Observation Schedule; ADDM: Autism and Developmental Disabilities Monitoring ASD, autism spectrum disorder; ARFID: avoidant/restrictive food intake disorder; BAMBI: Brief Autism Mealtime Behavior Inventory; BPFAS: Behavioral Pediatric Feeding Assessment Scale; BMI: body mass index; CDC: Child Development Clinic; CDRC: Child Developmental and Rehabilitation Center; CEBQ: Children’s Eating Behavior Questionnaire; DSM-5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; GERD: gastroesophageal reflux disease; GDD: global developmental delay; IQR: interquartile range; MCH-FS: Montreal Children’s Hospital Feeding Scale; MBQ: Mealtime Behavior Questionnaire; M-CHAT: Modified Checklist for Autism in Toddlers; RRB: repetitive, restrictive behavior; SCI: social communication interaction; SD: standard deviation; SPSS: Statistical Package for the Social Sciences
| References | ▴Top |
- Ramsay M, Martel C, Porporino M, Zygmuntowicz C. The Montreal Children's Hospital Feeding Scale: A brief bilingual screening tool for identifying feeding problems. Paediatr Child Health. 2011;16(3):147-e117.
doi pubmed - Nadon G, Feldman DE, Dunn W, Gisel E. Mealtime problems in children with autism spectrum disorder and their typically developing siblings: a comparison study. Autism. 2011;15(1):98-113.
doi pubmed - Schreck KA, Williams K. Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Res Dev Disabil. 2006;27(4):353-363.
doi pubmed - Margari L, Marzulli L, Gabellone A, de Giambattista C. Eating and mealtime behaviors in patients with autism spectrum disorder: current perspectives. Neuropsychiatr Dis Treat. 2020;16:2083-2102.
doi pubmed - Pinto-Silva R, Nunes Costa AM, Tello-Rodrigues I. Feeding problems in children with autism spectrum disorders: a systematic review. Speech Lang Hear. 2023;26(2):130-141.
doi - Kamal Nor N, Ghozali AH, Ismail J. Prevalence of overweight and obesity among children and adolescents with autism spectrum disorder and associated risk factors. Front Pediatr. 2019;7:38.
doi pubmed - Sathyabama R. Clinical characteristics and demographic profile of children with Autism Spectrum Disorder (ASD) at child development clinic (CDC), Penang Hospital, Malaysia. Med J Malaysia. 2019;74(5):372-376.
pubmed - Walensky RP, et al. Morbidity and mortality weekly report prevalence and characteristics of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2020 Surveillance Summaries Centers for Disease. MMWR Surveill Summ. 2023;72(2):1-14.
- van Dijk MWG, Buruma ME, Blijd-Hoogewys EMA. Detecting Feeding Problems in Young Children with Autism Spectrum Disorder. J Autism Dev Disord. 2021;51(11):4115-4127.
doi pubmed - Rogers S, Ramsay M, Blissett J. The Montreal Children's Hospital Feeding Scale: Relationships with parental report of child eating behaviours and observed feeding interactions. Appetite. 2018;125:201-209.
doi pubmed - Kang YQ, Teo CM, Tan ML, Aw MM, Chan YH, Chong SC. Feeding difficulties in Asian children with autism spectrum disorder. Pediatr Neonatol. 2022;63(1):48-56.
doi pubmed - Seiverling L, Towle P, Hendy HM, Pantelides J. Prevalence of feeding problems in young children with and without autism spectrum disorder: a chart review study. J Early Interv. 2018.
doi - Patton SR, Odar Stough C, Pan TY, Holcomb LO, Dreyer Gillette ML. Associations between autism symptom severity and mealtime behaviors in young children presented with an unfamiliar food. Res Dev Disabil. 2020;103:103676.
doi pubmed - Jaafar NH, Othman A, Majid NA, Harith S, Zabidi-Hussin Z. Parent-report instruments for assessing feeding difficulties in children with neurological impairments: a systematic review. Dev Med Child Neurol. 2019;61(2):135-144.
doi pubmed - Lukens CT, Linscheid TR. Development and validation of an inventory to assess mealtime behavior problems in children with autism. J Autism Dev Disord. 2008;38(2):342-352.
doi pubmed - DeMand A, Johnson C, Foldes E. Psychometric properties of the brief autism mealtime behaviors inventory. J Autism Dev Disord. 2015;45(9):2667-2673.
doi pubmed - Sharp WG, Burrell TL, Jaquess DL. The Autism MEAL Plan: a parent-training curriculum to manage eating aversions and low intake among children with autism. Autism. 2014;18(6):712-722.
doi pubmed - Benjasuwantep B, Rattanamongkolgul S, Ramsay M. The Thai version of the Montreal children's hospital feeding scale (MCH-FS): psychometric properties. J Med Assoc Thai. 2015;98(2):163-169.
pubmed - Gray HL, Chiang HM. Brief report: mealtime behaviors of Chinese American children with autism spectrum disorder. J Autism Dev Disord. 2017;47(3):892-897.
doi pubmed - Adams SN. Feeding and swallowing issues in autism spectrum disorders. Neuropsychiatr Dis Treat. 2022;18:2311-2321.
doi pubmed - Madra M, Ringel R, Margolis KG. Gastrointestinal issues and autism spectrum disorder. Psychiatr Clin North Am. 2021;44(1):69-81.
doi pubmed - Bandini LG, Anderson SE, Curtin C, Cermak S, Evans EW, Scampini R, Maslin M, et al. Food selectivity in children with autism spectrum disorders and typically developing children. J Pediatr. 2010;157(2):259-264.
doi pubmed - Kothari P, Tate A, Adewumi A, Kinlin LM, Ritwik P. The risk for scurvy in children with neurodevelopmental disorders. Spec Care Dentist. 2020;40(3):251-259.
doi pubmed - Musa H, Ismail, II, Abdul Rashid NH. Paediatric scurvy: frequently misdiagnosed. Paediatr Int Child Health. 2021;41(2):158-161.
doi pubmed - Sharma R, Ghimire S, Dhungel KU. Autism and Food Selectivity. Janaki Med Coll J Med Sci. 2020;8(1):64-74.
doi - Estes A, Olson E, Sullivan K, Greenson J, Winter J, Dawson G, Munson J. Parenting-related stress and psychological distress in mothers of toddlers with autism spectrum disorders. Brain Dev. 2013;35(2):133-138.
doi pubmed - Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord. 2013;43(3):629-642.
doi pubmed - Falk NH, Norris K, Quinn MG. The factors predicting stress, anxiety and depression in the parents of children with autism. J Autism Dev Disord. 2014;44(12):3185-3203.
doi pubmed - Abdul Halim NH, et al. Prevalence of child’s behavioural feeding problems, body mass index and mental health issues among parents and children with autism in Malaysia. Asian J Med Biomed. 2021;5(S1):39-46.
doi - Curtin C, Hubbard K, Anderson SE, Mick E, Must A, Bandini LG. Food selectivity, mealtime behavior problems, spousal stress, and family food choices in children with and without autism spectrum disorder. J Autism Dev Disord. 2015;45(10):3308-3315.
doi pubmed - Ward-horner, John & Sturmey and Peter. Component analysis of behavior skills. Behaviroral Interv. 2012;27(February):75-92.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
