| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Case Report
Volume 4, Number 4, December 2015, pages 189-192
Pulmonary Artery Aneurysm as a Clue to Behcet’s Disease in a 7-Year-Old Boy
Ruth Gussenhovena, b, Simon Robbena, e, Jannie Hofc, Francois Vermeulend, Gijs van Wellb
aDepartment of Radiology, Division of Pediatric Radiology, Maastricht University Medical Center (MUMC+), Maastricht, The Netherlands
bDepartment of Pediatrics, Division of Pediatric Infectious Diseases and Immunology, Maastricht University Medical Center (MUMC+), Maastricht, The Netherlands
cDepartment of Ear, Nose and Throat (ENT) Surgery, University Hospital Leuven (UZL), Leuven, Belgium
dDepartment of Pediatrics, Division of Pediatric Infectious Diseases, Maastricht University Medical Center (MUMC+), Maastricht, The Netherlands
eCorresponding Author: Simon Robben, Department of Radiology, Maastricht UMC+, PO Box 5800, 6212 AZ Maastricht, The Netherlands
Manuscript accepted for publication November 20, 2015
Short title: Pulmonary Artery Aneurysm
doi: http://dx.doi.org/10.14740/ijcp231w
| Abstract | ▴Top |
Pulmonary artery aneurysm is a rare entity with a wide variety of underlying pathophysiological mechanisms. We report a 7-year-old boy who initially presented with suspected Lemierre’s syndrome (tonsillitis and jugular vein thrombosis). However, follow-up imaging studies showed progression of the thromboembolic changes into multiple dural venous structures as well as an aneurysm of the pulmonary artery, without respiratory symptoms. This rare combination of symptoms eventually led to the diagnosis of Behcet’s disease. In this report, we focus on the atypical presentation, the evolution of the clinical and radiological features and discuss the (radiological) clues, which led to the diagnosis.
Keywords: Pulmonary artery aneurysm; Behcet’s disease; Lemierre’s syndrome; Child
| Introduction | ▴Top |
Pulmonary artery aneurysm (PAA) is a rare entity with a prevalence rate of approximately 1 in 14,000 necropsies [1]. PAA has a wide variety of causes and is generally classified into a mycotic aneurysm, which arises from a bacterial infection and is previously described in patients with endocarditis, patent ductus arteriosus and its secondary interventional treatment, or non-mycotic aneurysms which in turn are associated with a variety of diseases such as Behcet’s disease, connective tissue disorders, Hughes-Stovin syndrome, trauma, and pulmonary hypertension [2]. These two different types of aneurysms are difficult to distinguish from each other exclusively using imaging studies. However, distinguishing these different entities is important to prevent any delay in diagnosis and treatment. Both mycotic and non-mycotic PAA may be complicated by acute rupture, causing hemoptysis and uncontrollable bleeding. This is obviously associated with a high mortality rate [1, 2]. Urgent appropriate treatment of suspected PAA is recommended and usually consists of (partial) lobectomy or pneumonectomy [1, 2]. Although interventional treatment options are improving, they are technically not always possible to perform, for example due to the location of the aneurysm [2].
Behcet’s disease, a relatively frequent cause of non-mycotic PAA, is a chronic relapsing immune-mediated vasculitis [3, 4]. Behcet vasculitis is distinguished by the involvement of both venous as well as arterial vessels [4]. It is a systemic disease in which almost all organs may be involved, causing a wide variety of symptoms. Clinically, Behcet’s disease is characterized by the presence of oral ulcers in 95-100% of cases [3]. Other common symptoms are uveitis and genital ulcers. The diagnosis is based on clinical criteria, established by the International Study Group for Behcet’s disease, and a positive pathergy test, which is pustule formation 24 - 48 h after skin injury [3]. Histologic findings are usually non-specific. PAAs in Behcet’s disease are caused by inflammation of the vasa vasorum, which destruct the elastic fibers, resulting in dilatation of the vessel. PAA in Behcet’s disease is a well-known cause of morbidity and mortality [3, 5].
| Case Report | ▴Top |
We report the case of a 7-year-old boy of African descent with no remarkable previous medical history, who initially presented at a general hospital with tonsillitis for which he received amoxicillin. During antibiotic treatment however, fever persisted and he developed diplopia and amblyopia due to a nervus abducens paresis. Reassessment of the referral imaging studies showed opacification of bilateral mastoid processes and thrombosis of the proximal right jugular vein with thrombus extension into the right sigmoid and transverse sinus up to the confluens sinuum. The sagittal superior and left sigmoid sinus and transverse sinus were open. Based on this imaging study, the diagnosis of Lemierre’s syndrome was made, antibiotic treatment consisting of ceftriaxone and clindamycin was started and he was referred to a university medical center for further evaluation and multidisciplinary treatment.
A mastoidectomy on the right side was performed in our patient by the ENT surgeon and antibiotic treatment was continued with clindamycin. Ceftriaxone was switched to meropenem extending the spectrum of antibiotic coverage to Pseudomonas aeroginosa and anaerobe growing microorganisms because of persisting fever and suspected infectious thrombosis. However, high-grade fever persisted and neck stiffness appeared. Therefore, magnetic resonance imaging (MRI) of the brain was repeated which revealed progressive extension of thrombosis into the sinus sagittalis superior and the left transverse, sigmoid and sphenoparietal sinus (Fig. 1). No parenchymal injury or leptomeningeal enhancement was seen. Low molecular-weight heparin was started in therapeutic doses, measured by anti-Xa activity because of progressive thrombosis. Extensive analysis of coagulation pathways revealed no prothrombotic hematologic disorder.
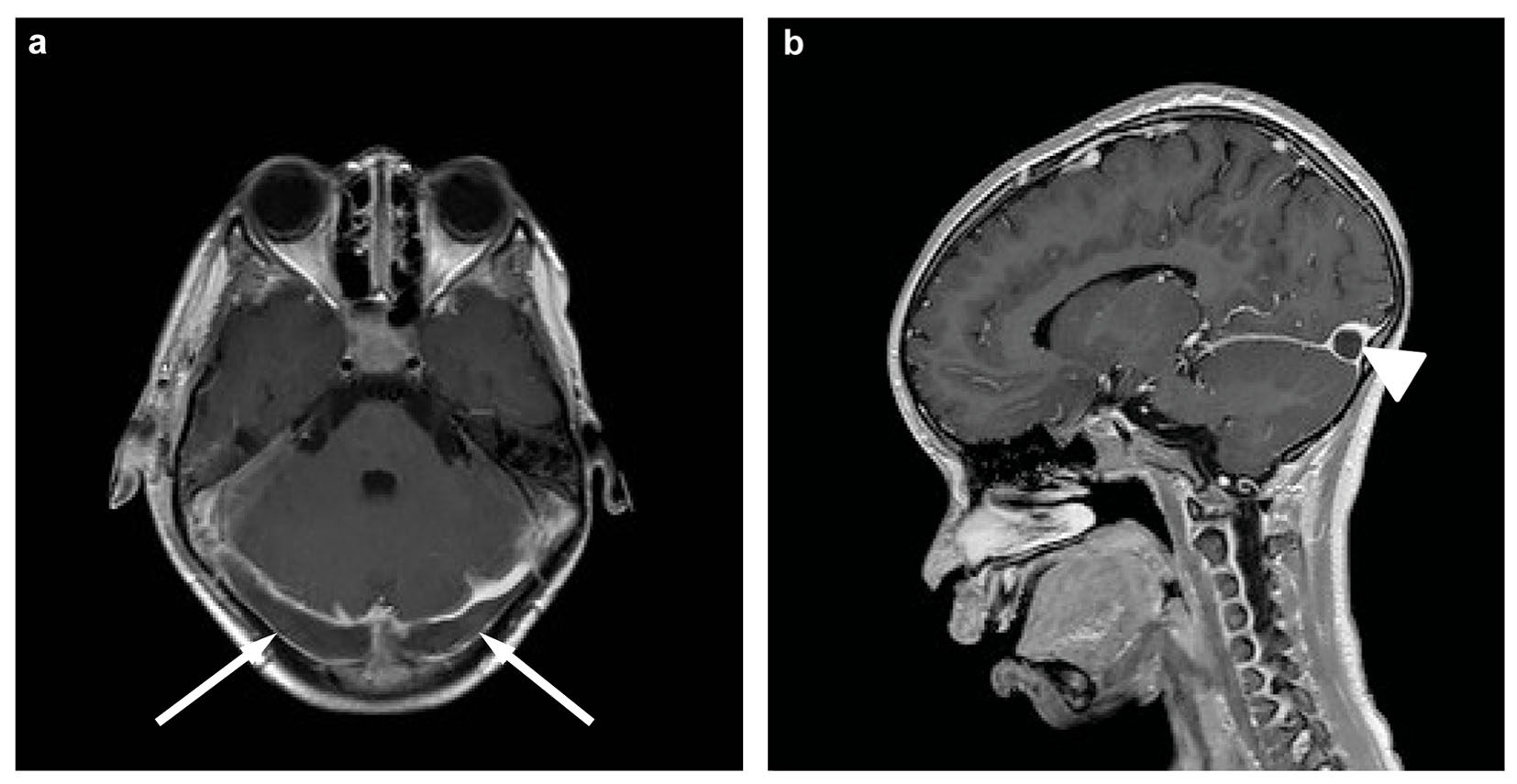 Click for large image | Figure 1. Magnetic resonance imaging of the brain, transverse (a) and sagittal (b) T1-weighted post-contrast. Both images show extensive thrombosis of the transverse sinus on both sides (arrows) and confluens sinuum (arrowhead). |
Although multiple blood samples were taken for blood culture, we never isolated Fusobacterium necrophorum or any other microorganism. The first blood culture was taken after antibiotic treatment was already started, and the patient continued antibiotic treatment for suspected Lemierre’s syndrome. Remarkably, follow-up studies revealed progression of the sinus thrombosis, despite adequate antibiotic and anticoagulation therapy. A partial thrombectomy was performed by the intervention radiologist, after which a slight decrease of sinus thrombosis was seen. After thrombectomy, the MRI showed stabilization of the thrombosis in size and location.
Because of persisting fever and progressive thrombosis under antibiotic and anticoagulative treatment, additional workup was performed including a thorax radiograph, bone scintigraphy, PET-CT scan, ultrasound of the abdomen, MRI of the brain, thorax and abdomen, which did not reveal any abnormalities, especially no abscesses, which could have been most likely expected in Lemierre’s syndrome.
One month after thrombectomy by catheterization, an ultrasound of the abdomen was performed because of abdominal pain, which revealed thrombosis of the right external and common iliac vein. This vein was used to perform the thrombectomy. On MRI, thrombosis of the right iliac vein was confirmed, extending to the infrarenal inferior vena cava. The left femoral vein showed normal flow voids. No evidence of local abscess was seen. This finding was considered to be contagious spread of infected thrombus during the interventional procedure. We also found a hyperintense lesion of the right lower lobe of the lung, which showed enhancement after contrast. Clinically, no pulmonary symptoms were noted. A subsequent CT scan showed opacification defects of the segmental branches of the right lower lobe matching multiple pulmonary emboli. Distal to a pulmonary embolus of the segmental branch of the dorso-basal segment of the right lower lobe, an aneurysmatic widening of the pulmonary artery was seen, which was partial filled with mural thrombus (Fig. 2a). The maximal diameter was 1.1 cm. This finding was considered to be a mycotic aneurysm due to septic emboli in a patient with Lemierre’s syndrome. The left lung did not show any pulmonary emboli. There were no parenchymal abnormalities of the lungs, especially no cavitations or abscesses. Echocardiography did not reveal any abnormalities.
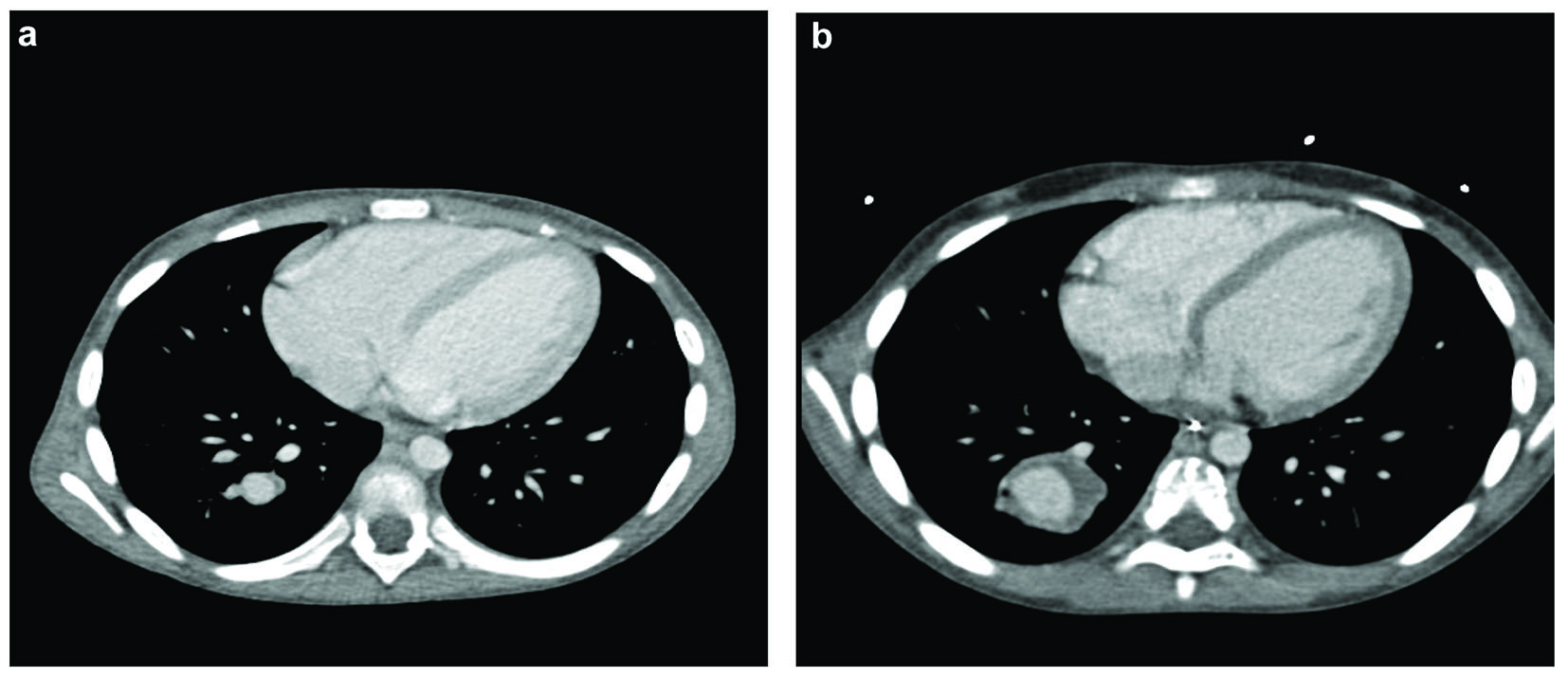 Click for large image | Figure 2. Contrast-enhanced CT of the thorax: (a) the initial size of the pulmonary artery aneurysm (diameter 1.1 cm), (b) the size of the aneurysm 1 month later (diameter 2.9 cm), also appreciate increase of the mural thrombus. |
Follow-up studies showed a progression in size of the aneurysm with a maximum diameter of 2.9 cm (Fig. 2b). Furthermore, new thrombosis of the left jugular vein, left brachiocephalic vein and superior vena cava was found, localized at the position of a central venous catheter. Induration of the surrounding fat of the left jugular vein was seen, which was considered a thrombophlebitis component in Lemierre’s syndrome. Follow-up studies, after removing the catheter, showed decreased size of the thrombosis, also the thrombosis of the femoral vein and inferior cava vein eventually decreased in size.
Endovascular techniques are preferentially used to manage PAA but considered impossible in this patient due to the peripheral location of the aneurysm. Therefore an indication for pulmonary lobectomy was set and the patient was referred to a university medical center for pediatric cardiothoracic interventions. The patient was transferred 3 months after initial presentation in the general hospital.
Post-operative histological examination of the resected lung tissue revealed an aneurysm of the pulmonary artery with destruction of the vessel wall and degradation of the lamina elastic interna and externa (Fig. 3). Furthermore, thrombus formation with an inflammatory infiltrate of macrophages, lymphocytes, plasma cells and neutrophilic granulocytes was present. Occasionally iron could be found. No eosinophils or giant cells were observed.
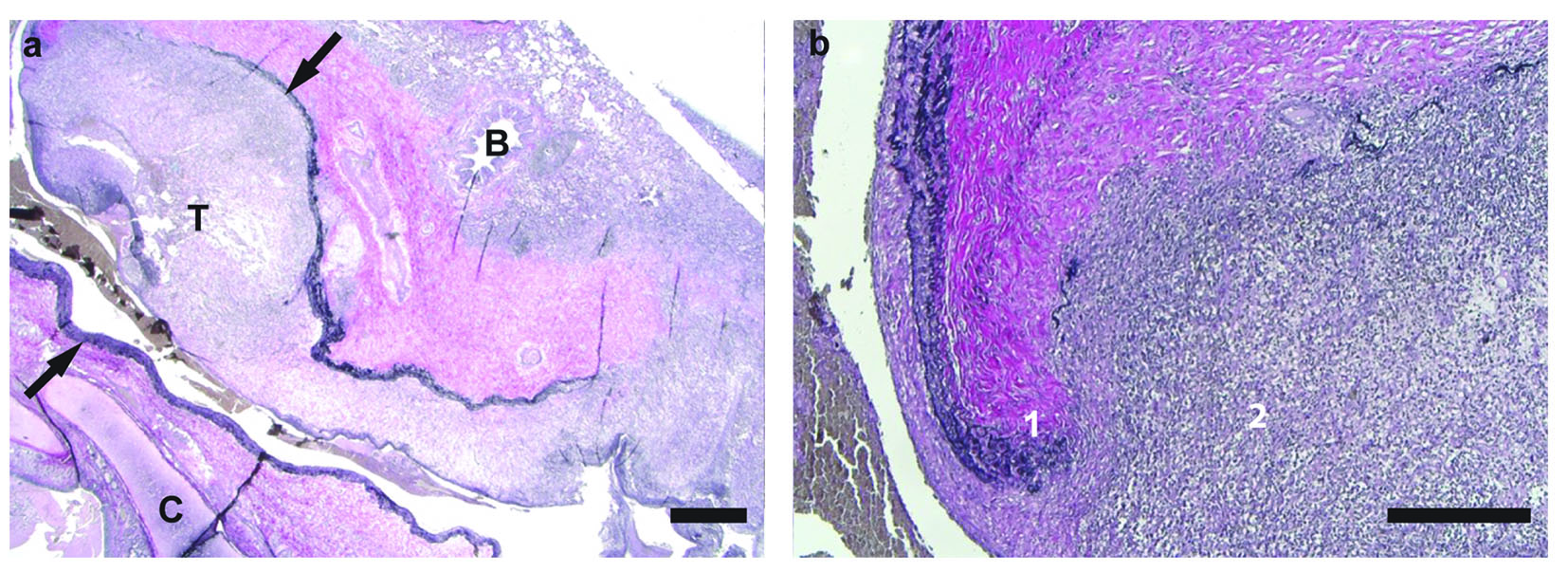 Click for large image | Figure 3. Elastica van Gieson stain of (a) pulmonary artery aneurysm (between arrows) with thrombus (T) (× 12 magnification; scale bar = 1,000 µm) and (b) pulmonary artery and inflammatory infiltrate (× 50 magnification; scale bar = 500 µm). There are (1) destruction of lamina elastica of vessel wall and (2) inflammatory infiltrate consisting of macrophages and neutrophilic granulocytes (T: thrombus; B: bronchus; C: cartilage). |
Based on the clinical history, imaging findings and pathological findings, the final diagnosis of Behcet’s disease was made. Anti-inflammatory treatment was started, consisting of pulses with high-dose corticosteroids followed by oral treatment with prednisolone and monthly cyclophosphamide. Currently, 1 year after initial presentation, the patient is doing well with low dose prednisolone and followed up by the pediatric rheumatologist.
| Discussion | ▴Top |
Initially, in our patient Lemierre’s syndrome was highly suspected considering the classical sequention of tonsillitis followed by thrombosis of the jugular vein and extensive sinus thrombosis. Lemierre’s syndrome is a suppurative thrombophlebitis of the jugular vein, which is frequently preceded by pharyngitis mostly with tonsillar or peritonsillar involvement. In extensive form, the infection may spread, causing systemic dissemination of septic emboli. The most frequently causative microorganism is Fusobacterium necrophorum [6].
However, there were several remarkable features, which made us reconsider this diagnosis. Firstly, no Fusobacterium necrophorum or any other microorganism was found in multiple cultures. Initially, these negative cultures were attributed to the fact that all cultures were taken after antibiotic treatment had already been started. Secondly, a hypercoagulable state persisted despite adequate anticoagulative treatment in the absence of a predisposing prothrombotic disorder. Thirdly, an aneurysmatic widening of the pulmonary artery has never been described in Lemierre’s syndrome. Aneurysmatic widening of an artery in Lemierre’s syndrome has only been described of the internal carotid artery and vertebral artery in two case reports [7, 8]. If the infection in Lemierre’s syndrome spreads systemically, the lung is the most commonly involved organ (80%) resulting in necrotic cavitary lesions, pleural effusion and lung abcesses [6, 8].
Moreover, the combination of venous as well as arterial lesions has not been described in Lemierre’s syndrome whereas this combination is distinctive for Behcet’s disease. Behcet’s disease is an autoimmune vasculitis, which does not differentiate between venous or arterial structures. PAAs have been previously described in Behcet’s disease and are an established cause of morbidity and mortality [3, 5]. Arterial involvement in Behcet’s disease is much less common than venous involvement and equates to 1-7% of the cases. If arterial aneurysms are present, the aorta is the most frequently affected followed by the pulmonary artery [3]. In addition, males are more likely to have arterial lesions than females and PAA involvement in Behcet’s disease is more frequently found in males (87%) [4, 5]. The mean age of PAA to occur is 29 ± 8 years [5]. The most common location of a PAA in Behcet is the right lower lobe concurrent to the location in our patient [3]. Cerebral venous thrombosis occurs in 5-25% of cases in Behcet’s disease. The most common sites of occlusion are the superior sagittal sinus, the transverse sinus, deep cerebral veins and the cavernous sinus [3]. Also the brain parenchyma can be involved and usually will be found in the brainstem [3].
Another noteworthy feature is that our patient originates from Africa whereas Behcet’s disease is most frequently reported in the eastern Mediterranean countries such as Turkey and the eastern part of Asia [3]. However, in the last years this has changed and prevalence in other parts of the world (e.g. North-America and Europe) is increasing [3].
In summary, Behcet’s disease is a clinical diagnosis for which international consensus criteria have been established but no specific histopathological test is available. The patient’s response to highly active anti-inflammatory treatment increases the likelihood of the diagnosis of Behcet’s disease in our patient. Retrospectively, our patient turned out to be known with oral ulcers at home in the years before hospital admission. This was not mentioned and not specifically asked during his first two hospital admissions. The tonsillitis, which our patient presented with, may be considered as a trigger for Behcet’s disease to flare.
Conclusion
Behcet’s disease is a difficult diagnosis. Early recognition of Behcet’s disease is important because regression of a PAA in Behcet’s disease is possible after corticosteroid or additive immunosuppressive treatment [3, 4]. In our case, early recognition and appropriate treatment might have prevented the invasive treatment that this patient eventually received.
Acknowledgement
We thank Dr. Carien Miedema, pediatrician in Catharina Hospital Eindhoven (CZE), and Carine Wouters, pediatrician in University Hospital Leuven (UZL), for excellent contribution to this case report. We thank Dr. Carine Peutz, pathologist in Maastricht UMC+ for excellent interpretation of the histology slides.
| References | ▴Top |
- Viart P, Cattelain C, Gallez A. Acquired pulmonary artery aneurysm in an infant. Pediatrics. 1980;65(1):89-93.
pubmed - Edwards S, Agrawal R, Donahoe N. Multiple mycotic pulmonary artery aneurysms. Publishing Endovascular Today. 2011. http://evtoday.com/2011/01/multiple-mycotic-pulmonary-artery-aneurysms/. Accessed 08 August 2014.
- Chae EJ, Do KH, Seo JB, Park SH, Kang JW, Jang YM, Lee JS, et al. Radiologic and clinical findings of Behcet disease: comprehensive review of multisystemic involvement. Radiographics. 2008;28(5):e31.
doi pubmed - Calamia KT, Schirmer M, Melikoglu M. Major vessel involvement in Behcet disease. Curr Opin Rheumatol. 2005;17(1):1-8.
doi pubmed - Seyahi E, Melikoglu M, Akman C, Hamuryudan V, Ozer H, Hatemi G, Yurdakul S, et al. Pulmonary artery involvement and associated lung disease in Behcet disease: a series of 47 patients. Medicine (Baltimore). 2012;91(1):35-48.
doi pubmed - Chirinos JA, Lichtstein DM, Garcia J, Tamariz LJ. The evolution of Lemierre syndrome: report of 2 cases and review of the literature. Medicine (Baltimore). 2002;81(6):458-465.
doi - Gutzeit A, Roos JE, Portocarrero-Fah B, Reischauer C, Claas L, Gassmann K, Hergan K, et al. Differential diagnosis of Lemierre's syndrome in a patient with acute paresis of the abducens and oculomotor nerves. Korean J Ophthalmol. 2013;27(3):219-223.
doi pubmed - Gupta T, Parikh K, Puri S, Agrawal S, Agrawal N, Sharma D, DeLorenzo L. The forgotten disease: Bilateral lemierre's disease with mycotic aneurysm of the vertebral artery. Am J Case Rep. 2014;15:230-234.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
