| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Original Article
Volume 13, Number 3, December 2024, pages 96-100
Children’s Peripheral Blood Leukocytes Immune Response on Some Brain and Nervous Tissue Antigens in Vitro
Svetlana Pleskanovskayaa, Muhammetaly Agamedovb, d, Nurgeldi Saparovc
aResearch Center, Myrat Garryev State Medical University of Turkmenistan, Ashgabat, Turkmenistan
bDepartment of Neurology and Neurosurgery, Myrat Garryev State Medical University of Turkmenistan, Ashgabat, Turkmenistan
cInternational Neurology Center, Ashgabat, Turkmenistan
dCorresponding Author: Muhammetaly Agamedov, Department of Neurology and Neurosurgery, Myrat Garryev State Medical University of Turkmenistan, Ashgabat, Turkmenistan
Manuscript submitted June 27, 2024, accepted August 24, 2024, published online December 2, 2024
Short title: Immune Response of Leukocytes to Brain Antigens
doi: https://doi.org/10.14740/ijcp546
| Abstract | ▴Top |
Background: There is a lack of information concerning the peripheral blood leukocytes sensibility on the nerve fibers and brain tissue antigens and their participation in the central nervous system (CNS) functional activity and pathology development. There is relevance of research aimed at the elaboration of the new biological markers of brain damage and nervous system diseases. Their introduction to CNS pathology diagnosis and treatment would lead to the development of new diagnostic and therapeutic strategies.
Methods: This investigation focused on the sensitivity of blood leukocytes to soluble nerve fiber and brain antigens in vitro among healthy children, as well as children with epilepsy and autism.
Results: It has been shown that, in vitro, some antigens of the brain and nervous tissue modulate (both inhibit and stimulate) the migration of the peripheral blood leukocytes from the glass capillary in practically healthy children. Consequently, in children without visible neurological symptoms, peripheral blood contains leukocytes that are specifically sensitized to these antigens of the brain and nervous tissue. In this regard, children with epilepsy were examined. At the beginning of treatment, the patients’ leucocyte sensibility to antigens significantly exceeded the reference values. During specific treatment, the indices decreased, and with a stable effect of anticonvulsant therapy, they practically returned to normal. In contrast, in children with autism, leukocyte migration in the presence of antigens was sharply reduced, compared to healthy children and children with epilepsy.
Conclusions: Significant fluctuations of the leucocytes’ response to the nervous system and brain antigens in vitro within a group of practically healthy children make us think about the presence of hidden neurological pathology in children. In addition, the obtained data, in our opinion, indicate the diagnostic value of changes in the degree of sensitization of blood leukocytes in CNS pathology.
Keywords: Nervous system pathology; Brain pathology; Immune diagnostics; Brain tissue antigen; Nervous tissue antigen; Leucocytes’ sensibilization to brain antigens
| Introduction | ▴Top |
Diseases affecting the nervous system are diverse and include nervous system development disorders, late age-related changes, neurodegeneration and cognitive impairment after infections. Total neurological loss, adjusted for years of disability, increased by 18.2% (from 375 million in 1990 to 443 million in 2021) [1-11]. The role of the immune system in neurological pathology remains unclear.
The immune system is usually viewed as autonomous. It was believed that the brain was protected from the immune system by blood-brain barrier. In recent decades, this paradigm has been seriously challenged by ample evidence that not only does the nervous system receive messages from the immune system, but also that brain signals regulate immune functions, which subsequently control inflammation in other tissues [2-6]. Consequently, in some neurological diseases, the blood-brain barrier is disrupted. As a result, there is an influx of cells of bone marrow origin into the central nervous system, which contributes to an increase in the pool of myeloid cells in it [3]. As a result, the risk of developing an auto-aggressive process in the tissues of the brain and nerve fibers increases.
Inflammation is a major contributor to the pathophysiology of various nervous system diseases. However, the innate immune system is an important mechanism that recruits microglia, resulting in clinical problems ranging from neurodevelopmental disorders to neurodegenerative diseases [4-12]. It has been shown that the severity of structural disorders in brain tissue is accompanied by an increasing concentration of neuronal cell damage markers and a decreasing concentration of astroglial cell markers [4, 5]. Markers of early brain damage include neuron-specific enolase, glial fibrillary acidic protein, sFasL and a number of others. Their level in the blood serum is considered as an indicator of cerebral pathology and its severity [3-6].
It has been established that in brain pathology (for example, ischemia) the neuronal and glial tissues cells death is the result of both necrosis and apoptosis. Inducers of apoptosis are immune system cells’ functional activity products such as tumor necrosis factor alpha (TNFα), interleukin (IL)-6, activated leukocyte cell adhesion molecule (ALCAM), glial protein S-100, myelin basic protein complex (MBP) and a number of other factors. Their appearance is a consequence of the reaction of immunocompetent cells [5-7]. Consequently, the death of neuronal and glial tissues has an immune nature.
The development of neuroimmunology contributes not only to a clearer understanding of neurological diseases’ pathogenesis. As a result of investigations in this direction, principally new methods for central nervous system damage diagnosis and prognosis have been developed [8, 9]. Directly or by implication, they indicate the immune system’s involvement in the pathogenesis of central nervous system (CNS) diseases [10, 11]. However, there is no information concerning the peripheral blood leukocytes sensibility on the nerve fibers and brain tissue antigens and their participation in CNS functional activity and pathology development. All of the above determines the relevance of research aimed at the elaboration of the new biological markers of brain damage and nervous system diseases. Their introduction to the diagnosis and treatment of CNS pathology could lead to the development of new diagnostic and therapeutic strategies.
The purpose of this work was to study the sensitivity of peripheral blood leukocytes on the brain and nervous tissues’ antigens in practically healthy children (PHC) and children with pathology of the nervous system aged 2 to 7 years.
| Materials and Methods | ▴Top |
We examined 135 PHC and 50 children with epilepsy (n = 20) and autism (n = 15), who were under observation at the International Neurology Center (Ashgabat). In both PHC and patients, in addition to special clinical and instrumental examinations which include a neurological exam, electroencephalogram (EEG), and magnetic resonance imaging (MRI) of the brain, the degree of peripheral blood leukocytes’ sensitivity on nerve fiber (N. ischiadicus) tissue antigen (NFTA) and the brain frontal lobe tissues’ antigen (BTA) was determined. The material for preparing the antigens was obtained from autopsies of males aged 20 to 25 years who died from accidental injury. The degree of sensitization of leukocytes to NFTA and BTA was determined in a modified reaction of leukocyte migration inhibition (RLMI) [12]. NFTA and BTA were used as migration inducers. Antigens were prepared by water-salt extraction method [13] following the recommendations [14]. Antigens were dosed according to protein concentration, which was determined by the Lowry method [15]. The protein concentration in the antigens was at least 20 µg/mL. The antigens (1.0 mL) were stored in disposable microtubes in a freezer at -20 °C. Antigens were melted once before the study. During the study, 0.05 mL of antigen was added to the incubation medium of the capillary incubation chambers. An equal amount of 0.9% sodium chloride solution was added to the control chamber. The number of the leukocytes that migrated into the control chamber (without antigens) was taken as 100%. In relation to this, the leukocyte migration index (LMI) was calculated. The study design is presented in Figure 1.
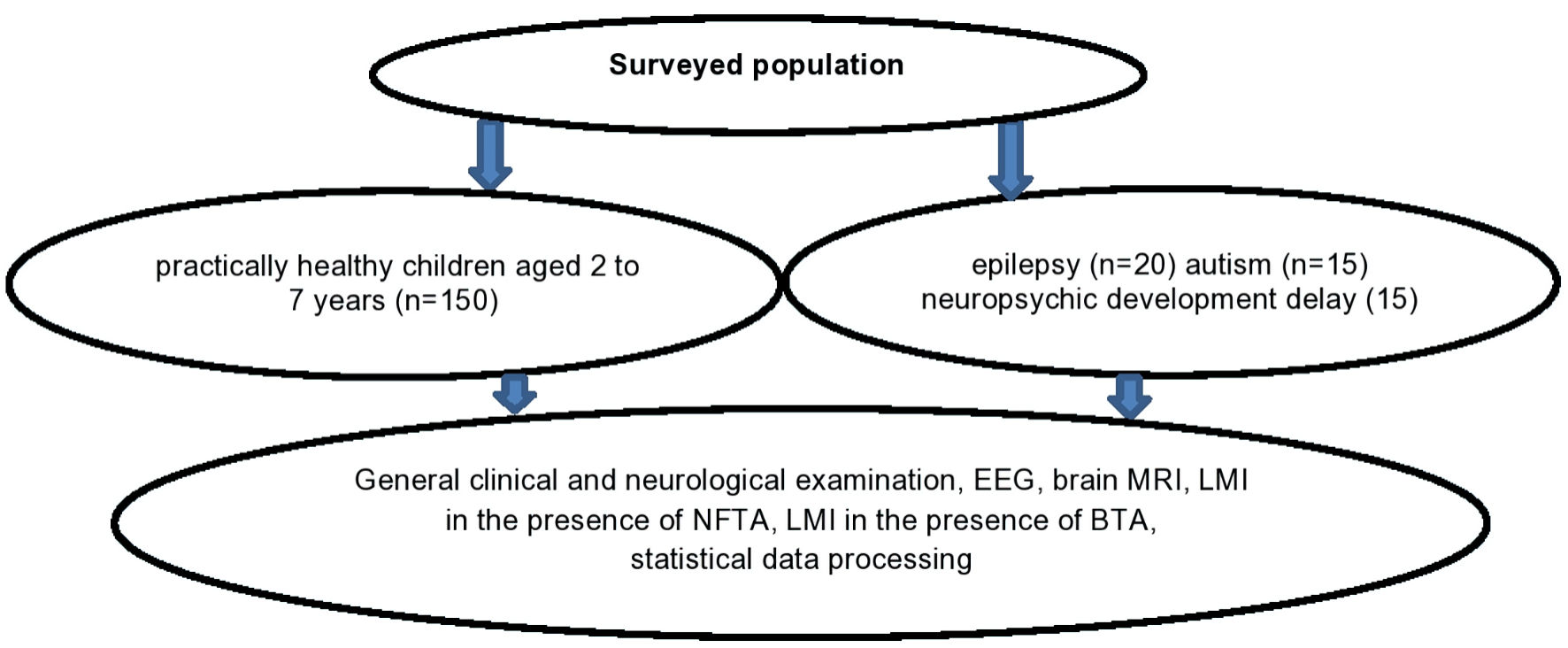 Click for large image | Figure 1. Study design. EEG: electroencephalogram; MRI: magnetic resonance imaging; NFTA: nerve fiber tissue antigen; BTA: brain tissue antigen; LMI: leukocyte migration index. |
The obtained results were mathematically processed using the SPSS program (IBM Corp., 2014, IBM SPSS Statistics for Windows, version 24.0, Armonk, New York: IBM Corp.).
Ethics approval
Approval was obtained from the local ethics committee (M. Garryyev State Medical University of Turkmenistan). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results and Discussion | ▴Top |
It has been shown that NFTA and BTA in vitro modulate the migratory activity of peripheral blood leukocytes in PHC aged 2 to 7 years. The value of LMI varied within quite significant limits from 35 to 120. On average for the group of PHC, LMI-NFTA and LMI-BTA were 80.9±4.4% and 72.9±5.9%, respectively. These meanings of LMI were taken as reference values. However, the frequency of occurrence of the LMI value corresponding to the reference values varied significantly depending on the gender of the child (Fig. 2).
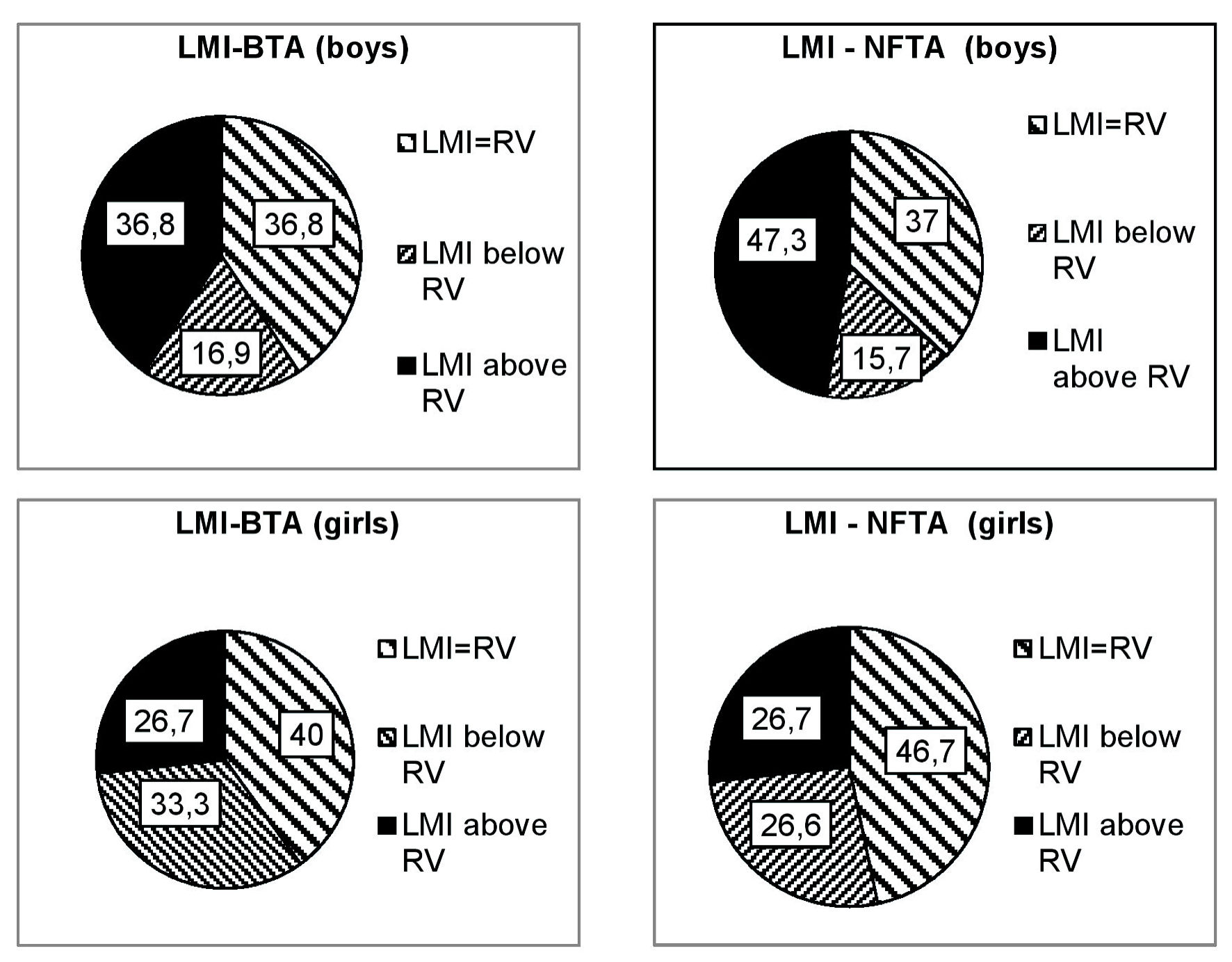 Click for large image | Figure 2. Frequency of children’s LMI value occurrence in % depending on antigen type and gender. NFTA: nerve fiber tissue antigen; BTA: brain tissue antigen; LMI: leukocyte migration index; RV: reference values. |
The diagram clearly shows that LMI-BTA, corresponding to the reference values for the group, occurred in almost equal numbers of cases in boys and girls (in 36.8 and 40% of cases, respectively). But in girls, low LMI-BTA occurred almost 15% more often, while high LMI-BTA was observed 10% less frequently in boys. LMI-BTA, exceeding the reference values, occurred almost 20% more often in boys than girls compared.
Thus, the peripheral blood leukocytes’ response to the brain and nervous tissue antigens in vitro of children without obvious pathology of the nervous system was almost the same (the difference is not significant, P > 0.05). However, a significant difference was revealed depending on the gender of the individuals examined. In girls, LMI-BTA was 73.1 ± 6.9; in boys it was 88.8 ± 7.6. LMI-NFTA in girls was 60.4 ± 5.7; in boys it was 85.5 ± 6.0. That is, the response of peripheral blood leukocytes to the brain and nerve fiber tissue antigens in boys is higher compared to girls of the same age. The difference is significant (P < 0.05 in both cases). Regardless of gender, there was a fairly strong direct correlation between LMI-BTA and LMI-NFTA (r = 0.59).
Thus, in vitro, some antigens of the brain and nervous tissue modulate (both inhibit and stimulate) the migration peripheral blood leukocytes from the glass capillary in PHC. Consequently, circulating leukocytes specifically sensitized to the antigens of brain and nervous tissue are present in the peripheral blood of children without visible neurological symptoms. Significant fluctuations in the value of LMI within a group of PHC make us think about the presence of hidden neurological pathology in children.
In this regard, 20 children suffering from epilepsy were examined. At initial treatment, the values of LMI-BTA and LMI-NFTA significantly exceeded the reference values and amounted on average for the group to 146.3 ± 10.1 and 119.3 ± 9.7, respectively. The difference was significant in relation to the PHC group (P < 0.01). In three cases out of 20, LMI-BTA was increased to 183, 190 and 220, respectively.
During specific treatment for 16 weeks, the indicators’ values decreased, and with a stable effect of anticonvulsant therapy, practically returned to normal (Fig. 3).
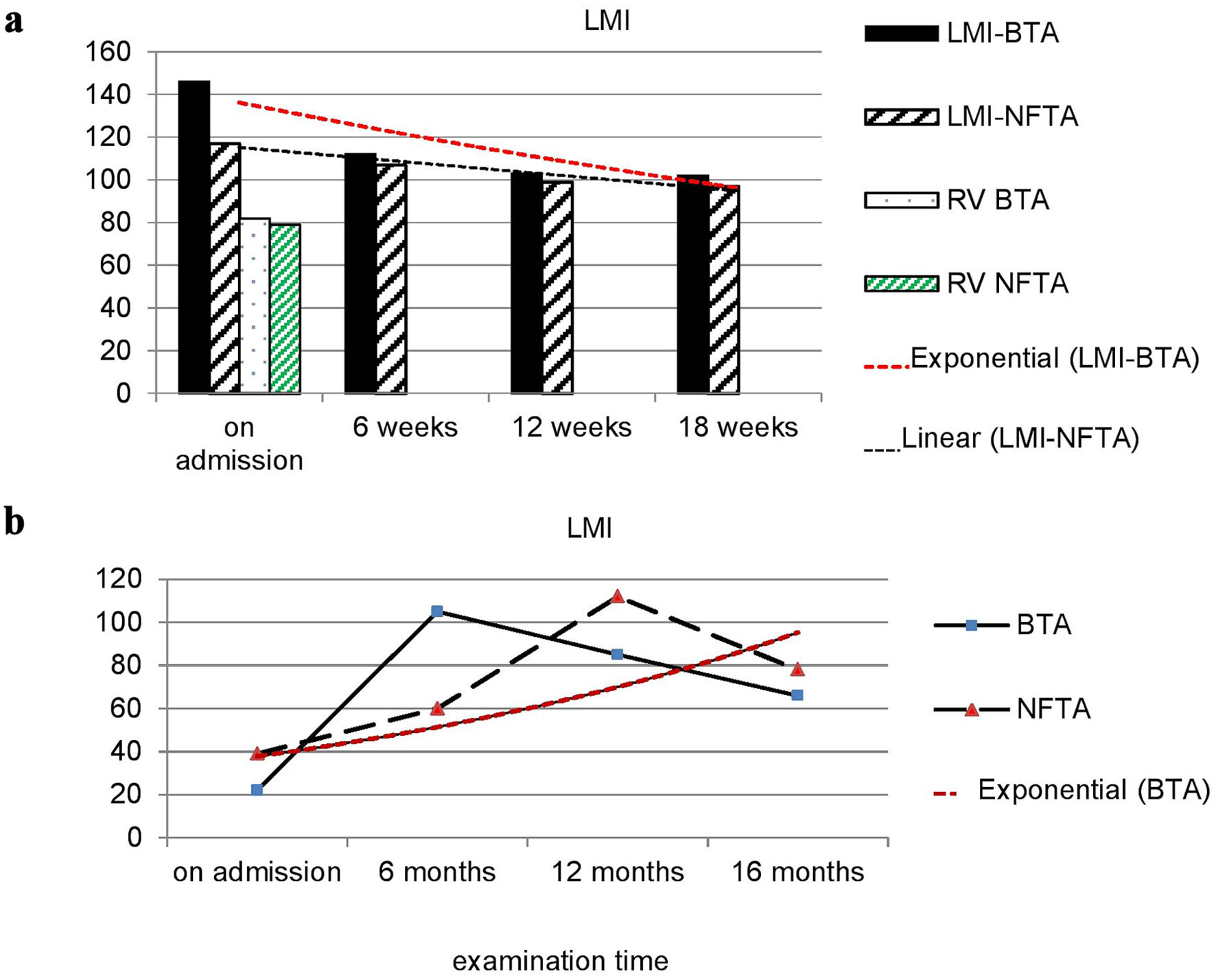 Click for large image | Figure 3. Dynamics of LMI-NFTA and LMI-BTA values in children with epilepsy (a) and autism (b). NFTA: nerve fiber tissue antigen; BTA: brain tissue antigen; LMI: leukocyte migration index; RV: reference values. |
In children with autism upon admission, the value of LMI-BTA and LMI-NFTA was significantly lower than the reference values and ranges for LMI-BTA from 17 to 32 (on average for the group 22.3 ± 1.7), and for LMI-NFTA from 19 to 40 (on average 39.3 ± 3.7) (Fig. 3b). The difference was significant in relation to the PHC group (P < 0.05). That is, in children with autism, in contrast to healthy children and children suffering from epilepsy, there is a sharp inhibition of leukocyte migration in the presence of antigens, and it is much more pronounced in the presence of a brain antigen compared to nervous tissue. Dynamic observation of children showed that after 6 months from the start of appropriate treatment, LMI-NFTA sharply increased against reference values and amounted to 112.7 ± 9.7. The difference was significant both in relation to the initial level and in relation to the PHC group (P < 0.01 and P < 0.05, respectively). The peak increase of LMI-BTA occurred at the 12th month of observation (Fig. 3b). By the 16th month of appropriate treatment, both indicators’ values were practically normalized.
Previously, the results of more than 13,000 studies showed that the degree of sensitization of the body to tissue antigens, expressed by the size of the pool of informed immunocompetent cells (ICC), is directly dependent on the type of tissue, the sex and age of the patient, the load on the organ and its functionality [16, 17]. In this regard, it is possible to assume the participation of sensitized leukocytes in both the peripheral and central nervous systems diseases pathogenesis. Of course, it is advisable to determine the degree of sensitization of circulating leukocytes to the antigens of all brain parts, both in healthy individuals and in patients suffering from CNS diseases.
Conclusions
The neuroimmunology development contributes to a clearer understanding of the pathogenesis of neurological diseases. As a result, in this direction, new methods for diagnosing and predicting central nervous system damage have been elaborated. Directly or by implication, they indicate the immune system’s involvement in the pathogenesis of CNS diseases. However, there is no information concerning the peripheral blood leukocytes sensibility on the nerve fibers and brain tissue’ antigens and sensibilized leucocytes’ participation in CNS functional activity and pathology development. In our opinion, there is relevance of research aimed at the elaboration the new biological markers of brain damage and nervous system diseases. Their introduction to CNS pathology diagnostics and treatment would lead to the development of new diagnostic and treatment strategies.
It has been shown, that in vitro, some antigens of the brain and nervous tissue modulate (both inhibit and stimulate) peripheral blood leukocytes migration from the glass capillary in PHC. Consequently, in the peripheral blood of children without visible neurological symptoms, circulating leukocytes specifically sensitized to antigens of brain and nervous tissue are present.
Further research in this direction is certainly relevant and will be continued by us.
Acknowledgments
None to declare.
Financial Disclosure
The authors received no financial support from any funding agencies for this study.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
SP: conceptualization, methodology, project administration, supervision, and writing the original draft. MA: data curation, data collection, and editing. NS: review and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
IS: immune system; PHC: practically healthy children; NFTA: nerve fiber tissue antigen; BTA: brain tissue antigen; LMI: leukocyte migration index; RLMI: reaction of leukocytes migration inhibition; CNS: central nervous system; RV: reference value; ICC: immunocompetent complex
| References | ▴Top |
- Huang Y, Li Y, Pan H, Han L. Global, regional, and national burden of neurological disorders in 204 countries and territories worldwide. J Glob Health. 2023;13:04160.
doi pubmed - Terrone G, Salamone A, Vezzani A. Inflammation and Epilepsy: Preclinical Findings and Potential Clinical Translation. Curr Pharm Des. 2017;23(37):5569-5576.
doi pubmed - Dalmau Gasull A, Glavan M, Samawar SKR, Kapupara K, Kelk J, Rubio M, Fumagalli S, et al. The niche matters: origin, function and fate of CNS-associated macrophages during health and disease. Acta Neuropathol. 2024;147(1):37.
doi pubmed - Herz J, Filiano AJ, Wiltbank AT, Yogev N, Kipnis J. Myeloid Cells in the Central Nervous System. Immunity. 2017;46(6):943-956.
doi pubmed - Morgun AB, Ovcharenko NV, Taranushenko TE, Ustinova SI, Okuneva OS, Antonova SK, Gilyazova DF, et al. Apoptosis markers and neurospecific proteins in diagnostics perinatal lesions of the central nervous systems in newborns. Siberian medical review. 2013;3(81):3.
- Taranushenko TE, Okuneva OS, Demyanova IM, Salmina AB, Mali-novskaya NA, Sharoglazova LA, Kritskaya IA, Morgun AV. Protein levels of neuronal and glial nature in the blood of newborns with cerebral is-chemia. Pediatrics. Journal named after G.N. Speransky. 2010;89(1):25-30.
- Mutalov AG, Greshilov AA, Amirova VR. Neuroimmunological diag-nostic criteria and prediction of perinatal lesions central nervous system in newborns. Medical Bulletin of Bashkortostan. 2010;5(1):34-39
- Kovtun OP, Gromada NE. Peculiarities cellular energy metabolism, im-munological and neurobiochemical diagnostic criteria perinatal hypoxic lesions central nervous system in newborns. Russian Bulletin of Perina-tology and Pediatrics. 2012;57(4-2):26-32.
- Noe FM, Polascheck N, Frigerio F, Bankstahl M, Ravizza T, Marchini S, Beltrame L, et al. Pharmacological blockade of IL-1beta/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis. 2013;59:183-193.
doi pubmed - Zhang W, Xiao D, Mao Q, Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. 2023;8(1):267.
doi pubmed - Shi M, Chu F, Zhu F, Zhu J. Peripheral blood amyloid-beta involved in the pathogenesis of Alzheimer's disease via impacting on peripheral innate immune cells. J Neuroinflammation. 2024;21(1):5.
doi pubmed - Pleskanovskaya SA. Cellular and humoral immune response in cutaneous leishmaniasis (experimental research and observations) Author's abstract. Diss. Cand. Honey. Sci. Moscow-1992. 22 p.
- Frimel G., Immunological Methods, Moscow, Meditsina, 1987, p. 321.
- Reed-Geaghan EG, Croxford AL, Becher B, Landreth GE. Plaque-associated myeloid cells derive from resident microglia in an Alzheimer's disease model. J Exp Med. 2020;217(4):e20191374.
doi pubmed - Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265-275.
pubmed - Pleskanovskaya SA, Orazaliev AS. Osteopathy (immunological aspects of diagnosis, treatment and prevention), 2018, LAP – LAMBERT (Academic Publishing), p. 51.
- Pleskanovskaya SA, Mamedova G, Ozturk M, Gucel S, Ashyraliyeva M. An overviev of the ethnobotany of Turkmenistan and use of Juniperus Turcomanica in phytotherapy. In: Singh RJ, Ed. Genetic resources, chromosome engineering, and crop improvement (Medical plants). Vol. 6, chapter 8. CRC Press, Taylor & Francis Group, Bocf Raton-London-New York; 2012. p. 207-220.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
