| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Case Report
Volume 13, Number 3, December 2024, pages 105-109
Pericardial Decompression Syndrome in a Pediatric Patient
Monette Verala, Anisha Contractorb, Majid Husainb, Ivanna Maxsonc, Micah Kaddenc, d
aDepartment of Pediatrics, Mattel Children’s Hospital, University of California-Los Angeles, Los Angeles, CA, USA
bDepartment of Pediatrics, Division of Pediatric Cardiology, Mattel Children’s Hospital, University of California-Los Angeles, Los Angeles, CA, USA
cDepartment of Pediatrics, Division of Pediatric Critical Care Medicine, Mattel Children’s Hospital, University of California-Los Angeles, Los Angeles, CA, USA
dCorresponding Author: Micah Kadden, Department of Pediatrics, Division of Pediatric Critical Care Medicine, Mattel Children’s Hospital, University of California-Los Angeles, Los Angeles, CA, USA
Manuscript submitted April 10, 2024, accepted June 8, 2024, published online July 10, 2024
Short title: Pediatric PDS
doi: https://doi.org/10.14740/ijcp536
| Abstract | ▴Top |
Pericardial decompression syndrome (PDS) is a rare but critical complication of pericardial drainage. It is most associated with pericardial drainage to treat cardiac tamponade but can also occur without preceding tamponade physiology. Pericardial drainage is typically performed to relieve external compression on the heart in order to help restore normal cardiac function. In PDS, patients paradoxically develop hemodynamic instability and pulmonary edema from ventricular dysfunction after drainage of a pericardial effusion. A 4-year-old boy with history of chronic granulomatous disease (CGD) who ultimately underwent haploidentical hematopoietic stem cell transplant (HSCT) required prolonged hospitalization for multiple infections and graft-versus-host disease. His clinical course was complicated by the development of a pericardial effusion for which he underwent pericardiocentesis. Shortly after the procedure, the patient developed worsening hemodynamics and pulmonary edema. Workup revealed new-onset right ventricular failure which was ultimately attributed to PDS. This case highlights PDS as a potential risk associated with pericardiocentesis in pediatric patients.
Keywords: Pericardial decompression syndrome; Pericardial drainage; Pericardiocentesis; Post-pericardiotomy low cardiac output syndrome
| Introduction | ▴Top |
Pericardial decompression syndrome (PDS) is the paradoxical worsening of hemodynamics and/or development of pulmonary edema related to ventricular dysfunction after an uncomplicated pericardiocentesis [1, 2]. It can present with isolated left ventricular (LV), right ventricular (RV), or biventricular failure [1-4]. While PDS is most commonly associated with pericardial drainage for cardiac tamponade, it can also occur in cases without tamponade [1]. It is critical that intensivists and cardiologists be familiar with this diagnosis and its typical presentation, proposed pathophysiology, and management.
We describe a case of PDS in a 4-year-old male who underwent pericardiocentesis for management of pericardial effusion. The patient’s parents provided consent for this case report.
| Case Report | ▴Top |
A 4-year-old boy with history of chronic granulomatous disease (CGD) who underwent haploidentical HSCT with successful engraftment remained admitted post-transplant for multiple transplant-related complications including cytomegalovirus (CMV) viremia and graft-versus-host disease. History was notable for the patient being born in Venezuela. He had been developmentally appropriate and healthy until the age of 12 months; around that age he began to suffer from recurrent infections including otitis media, phlebitis, and adenitis. On multiple occasions, his adenitis was treated with drainage; cultures of this fluid grew multiple opportunistic microbes including Candida krusei, Burkholderia cepacia, and Acinetobacter baumannii. At 18 months of age after extensive workup, he was ultimately diagnosed with X-linked CGD. At that time, he was started on prophylactic antimicrobials, but continued to suffer from recurrent infections. At 2 years of age, his family immigrated to the United States where he established ongoing care. He ultimately required a haploidentical HSCT and remained admitted to the pediatric bone marrow transplant service for ongoing management. His initial post-transplant course was notable for the development of refractory CMV viremia and graft-versus-host disease.
On day 105 post-transplant, the patient was noted to have worsening clinical status and a rapid response was called. At that time, his temperature was 37.7 °C, heart rate was 137, respiratory rate was 45, blood pressure was 109/65 mm Hg, and oxygen saturation was 98% on 6 L of nasal cannula. On exam, the patient was noted to have moderate increased work of breathing with subcostal retractions, diffuse crackles were appreciated on auscultation. From a cardiac perspective, heart sounds were normal, strong pulses were appreciated in all four extremities, and capillary refill was noted to be 2 - 3 s. Neurologically, the patient was alert, answered questions appropriately, and did not have any focal deficits. Labs were notable for white blood cell count of 1.96 × 103/µL, hemoglobin of 8.8 g/dL, and a platelet count of 37 × 103/µL. Electrolytes were within normal limits. CMV polymerase chain reaction (PCR) level in the blood was 458,000 IU/mL, down from a peak of 1,780,000 IU/mL 2 weeks prior. A chest X-ray (CXR) demonstrated diffuse mild pulmonary edema, bilateral pleural effusions, and an enlarged cardiac silhouette. A computed tomography (CT) scan of the chest was obtained and showed bilateral diffuse airway opacities with mild-to-moderate sized simple pleural effusions, and a moderate-to-large pericardial effusion.
He was diagnosed with respiratory failure with concern for sepsis and was transferred to the pediatric intensive care unit (PICU) for ongoing management. He was escalated to high-flow nasal cannula (HFNC) and was started on meropenem. Despite ongoing management with foscarnet, there was concern for refractory CMV infection; maribavir and cidofovir were added. Given his respiratory decompensation and findings on CT of bilateral simple pleural effusions, the patient was started on a bumetanide drip. Chest tube placement was deferred while attempting to treat the pleural effusions conservatively with diuresis. He underwent bronchoscopy with bronchoalveolar lavage (BAL); CMV was detected in the BAL fluid confirming a diagnosis of CMV pneumonitis. Bacterial and fungal cultures obtained during the BAL resulted as negative; additionally, multiple opportunistic infections were tested for and resulted as negative.
Given the findings of pericardial effusion on the CT scan, an echocardiogram was obtained and confirmed a qualitatively small-to-moderate circumferential pericardial effusion with the largest diameter measuring 10 mm but without evidence of cardiac tamponade. This was suspected to be a chronic inflammatory pericardial effusion. Given these findings and the fact that his CMV treatment had been optimized, pericardial drainage was deferred with plan to monitor the patient clinically and repeat serial echocardiograms.
One week after transfer to the PICU, the patient’s blood CMV titers were improving (23,800 IU/mL). His pleural effusions had improved with conservative management on the bumetanide drip. Despite this, he remained on HFNC and interval echocardiogram redemonstrated the circumferential pericardial effusion with an increase in the size at its largest diameter to 18 mm. Two weeks later, now 125 days post-HSCT, his clinical status worsened. His vitals at the time were temperature of 38.7 °C, heart rate was 180, respiratory rate was 40, blood pressure was 92/35 mm Hg, and oxygen saturation was 90% on 40 L of HFNC with an FiO2 of 100%. On exam, he was visibly exhausted but neurologically appropriate and interactive without focal neurologic deficits. He was tachypneic and dyspneic with subcostal and suprasternal retractions. He was noted to have diffuse coarseness with crackles throughout his bilateral lung fields. His pulses were strong in all four extremities and capillary refill was 2 - 3 s. Heart sounds were normal. All other components of the exam were non-contributory. CXR demonstrated diffuse haziness without focality with small bilateral pleural effusions.
Labs at that time were notable for a white blood cell count of 0.54 × 103/µL, hemoglobin of 9.6 g/dL, and a platelet count of 36 × 103/µL. Other than an elevated blood urea nitrogen (BUN) (27 mg/dL) which was likely related to the patient’s ongoing diuresis, electrolytes were within normal limits. CMV PCR level in the blood was down to 7,560 IU/mL.
Given his worsening cardio-respiratory status, the patient was intubated. Post-intubation, the patient was sedated with fentanyl and dexmedetomidine drips. He remained hypoxic on moderate ventilatory support. Notably, despite adequate sedation, fever control, and fluid resuscitation, he remained tachycardic with heart rates in the 160s. A repeat echocardiogram was obtained and the circumferential pericardial effusion now measured 19 mm at its largest diameter (Fig. 1). The patient was noted to have good biventricular function and there was no evidence of tamponade physiology. There was considerable debate regarding the best approach to managing the pericardial effusion as typical threshold for intervention includes evidence of cardiac tamponade and/or measuring greater than 20 mm. The size of the patient’s pericardial effusion put it on the cusp of warranting drainage. Additionally, given that the pericardial effusion continued to increase in size and there was concern that it contributed to his decompensation, decision was made to proceed with pericardiocentesis. Additionally, the procedure allowed for laboratory analysis of the pericardial fluid.
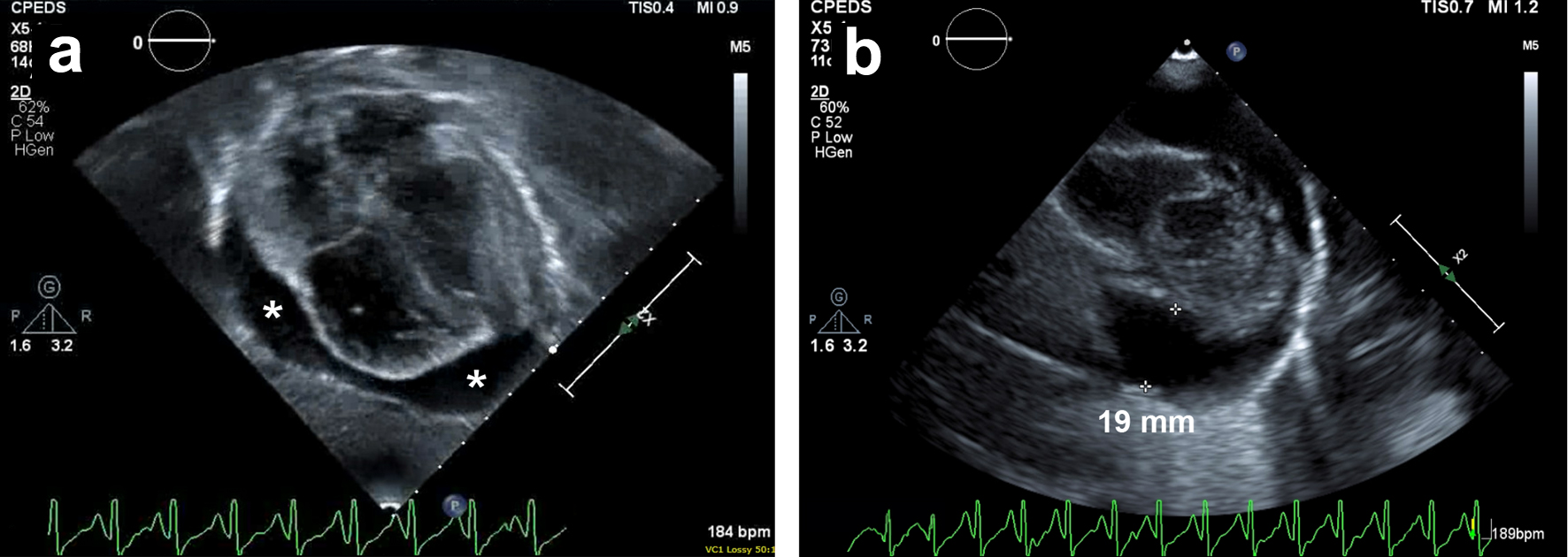 Click for large image | Figure 1. Transthoracic echocardiogram. (a) Subcostal and (b) parasternal short axis views demonstrating a moderate-sized circumferential pericardial effusion measuring 19 mm at its largest depth. * denotes areas of pericardial effusion. |
Pericardiocentesis was performed and approximately 200 mL of serous fluid was immediately drained and a pericardial drain was left in place. Post-procedure his tachycardia improved with heart rates ranging between 100 and 115, and a repeat echocardiogram showed resolution of the pericardial effusion and normal biventricular function. The pericardial fluid was straw colored. Lab studies on the pericardial fluid were notable for a total protein of 4.4 g/dL, lactate dehydrogenase 334 U/L, and glucose of 122 mg/dL. Cultures were also sent and resulted as negative. CMV PCR on the pericardial fluid was not detected which reassured against active CMV infection within the pericardium. Overall, these findings were consistent with an inflammatory pericardial effusion secondary to the patient’s overall clinical state.
Approximately 6 h after the pericardiocentesis, the patient developed acute hypotension with blood pressures of 50s/30s mm Hg and tachycardia to the 170s. He then developed severe bradycardia with a heart rate in the 40s. He required resuscitation with multiple epinephrine boluses and ultimately required high-dose epinephrine, norepinephrine, and vasopressin infusions to maintain appropriate blood pressure for age, additionally he was started on stress dose hydrocortisone. He required increased respiratory support on the ventilator and a repeat CXR was notable for mildly increased diffuse pulmonary infiltrates consistent with worsening pulmonary edema. Infectious workup was obtained and antimicrobials were broadened to include meropenem, linezolid, and amphotericin B, given concern for septic shock. Echocardiogram showed a dilated RV with interventricular septal flattening. The RV function was severely diminished and there was moderate tricuspid valve regurgitation with an estimated right ventricular systolic pressure (RVSP) that was greater than half systemic (Fig. 2). Despite the flattened interventricular septum, LV function was preserved. Notably, there was no evidence of pericardial effusion. Inhaled nitric oxide was initiated for RV afterload reduction and he continued on high-dose epinephrine, norepinephrine, and vasopressin drips. Within 24 h, he was weaned off all vasoactive medications and a follow-up echocardiogram demonstrated spontaneous improvement in RV function: the RV was mildly dilated with qualitatively moderately diminished systolic function, mild tricuspid valve regurgitation, and an estimated RVSP less than a third systemic. He was then extubated to HFNC and inhaled nitric oxide was discontinued the following day. The pericardial drain was removed 3 days after initial placement and repeat echocardiogram demonstrated normal biventricular function without pericardial effusion. Of note, blood cultures obtained around the time of his decompensation were negative and the linezolid and meropenem were discontinued. Aspergillus galactomannan antigen enzyme immunoassay (EIA) from the serum resulted as positive with a value of 3.26 EIA units. The patient was continued on amphotericin B.
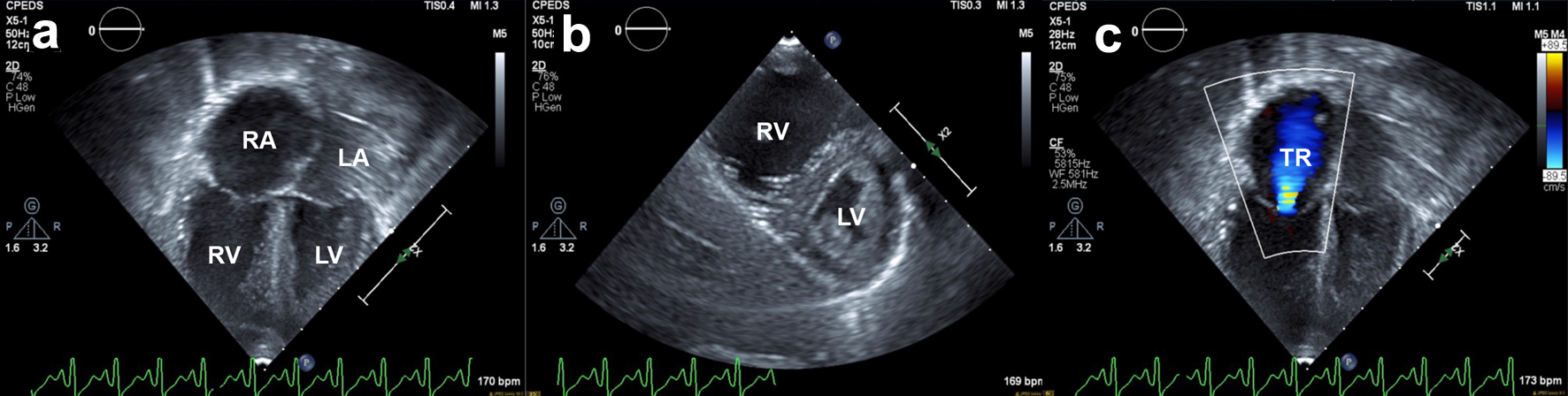 Click for large image | Figure 2. Transthoracic echocardiogram. Still frames obtained from 2D cine clips. (a) Apical four-chamber view: severe RV dilation and reduced systolic RV function. Normal left ventricular systolic function. (b) Parasternal short axis: interventricular septum flattening through the cardiac cycle, more pronounced in diastole, suggestive of RV volume overload. (c) New-onset moderate tricuspid regurgitation secondary to RV dilation and ventricular dysfunction. RA: right atrium; LA: left atrium; RV: right ventricle; LV: left ventricle; TR: tricuspid regurgitation. |
Over the 3 weeks following the patient’s recovery from PDS, he slowly clinically deteriorated. He developed worsening respiratory failure followed by the development of septic shock. His Aspergillus galactomannan antigen EIA remained positive despite treatment with amphotericin B; anti-fungal coverage was expanded to include micafungin. He was intubated and restarted on vasoactive medications for septic shock. He was also restarted on empiric antibiotics with linezolid and meropenem. Blood cultures obtained were positive for Enterococcus faecalis; linezolid and meropenem were discontinued and he was started on ampicillin. The patient progressed to multi-organ failure and suffered from a cardiac arrest. Resuscitation efforts were initiated in line with the wishes of the family, but were unsuccessful and the patient died.
| Discussion | ▴Top |
We describe a case of PDS in a pediatric patient shortly after pericardiocentesis for pericardial effusion. Our patient was medically complex with a history of CGD, and he ultimately underwent HSCT. His post-transplant course was complicated by the development of refractory CMV viremia. The course was further complicated by the development of an inflammatory pericardial effusion. After initial conservative management, the decision was made to perform a pericardiocentesis. The patient clinically deteriorated shortly after the procedure and was ultimately diagnosed with PDS. He was treated with supportive management and his PDS resolved within 24 h. Unfortunately, despite recovering from his PDS, the patient later developed septic shock and multi-organ failure and eventually died.
PDS is an acute hemodynamic sequela of pericardiocentesis or surgical pericardiostomy that carries significant risk of morbidity and mortality [1]. While there are many reports of PDS in adults [1], there is only one reported pediatric case, which occurred in a teenage patient with a large pericardial effusion and cardiac tamponade prior to pericardial drainage [5]. We present a novel pediatric case of a 4-year-old male who developed PDS after pericardiocentesis of a moderate-sized chronic inflammatory pericardial effusion.
PDS has been reported to occur immediately after pericardial drainage but can present up to 48 h after [3]. A recent study that analyzed 62 cases of PDS in adults found that it can occur after a broad range of drained pericardial volumes (100 - 2,760 mL) [1]. Notably, this study also found that 4.8% of patients did not have cardiac tamponade prior to undergoing pericardial drainage [1]. PDS can be fatal; in cases of survival, recovery of ventricular function ranges from 1 to 21 days [1].
The mechanism of PDS is not well understood. There are three proposed pathophysiological hypotheses: hemodynamic, ischemic, and autonomic. The hemodynamic hypothesis proposes increased systemic venous return to a more compliant RV after pericardiocentesis, which results in increased RV preload and RV dilation [2, 6, 7]. This can lead to RV failure and LV compression from interventricular septum bowing, which limits LV preload leading to decreased cardiac output and pulmonary edema [2, 6, 7]. The cardiac output is further decreased due to increased systemic resistance which develops during the state of cardiac tamponade but is thought to persist after pericardial drainage [2, 6]. The ischemic hypothesis relies on the premise that cardiac tamponade causes decreased coronary artery perfusion and in turn causes cardiac ischemia [8]. The cardiac ischemia leads to ventricular stunning that persists after pericardiocentesis and restoration of coronary blood flow [2, 6]. This theory relies on an initial state of cardiac tamponade and may not be as applicable to drainage of chronic pericardial effusions that are not associated with tamponade. The autonomic hypothesis suggests that during the state of cardiac tamponade, the body compensates with increased catecholamine levels to maintain cardiac output via chronotropy and inotropy [2, 9, 10]. Once pericardiocentesis is performed, there is no longer a driver of the increased catecholamine state in the body [2, 9, 10]. This uncovers myocardial dysfunction that was compensated for in the high catecholamine environment [2, 9, 10]. Our patient’s PDS presentation with RV failure with echocardiogram findings at the time of diagnosis significant for RV dilation and septal bowing seems to be most consistent with the hemodynamic hypothesis.
Little guidance on the prevention of PDS exists. The 2015 European Society of Cardiology guidelines provide a framework for management of adults with pericardial effusion, and it includes a recommendation of limiting pericardial fluid removal to less than 1 L in order to limit the risk of PDS [11]. Other adult studies propose limiting fluid removal to the volume required to relieve evidence of cardiac tamponade after which a pericardial drain should be left in place and subsequent fluid removal should occur gradually over days [6, 7]. This proposed treatment guideline has limited benefit when pericardial drainage is not associated with cardiac tamponade.
A critical aspect of management after pericardiocentesis includes hemodynamic monitoring ideally in an intensive care unit which allows early detection and treatment of PDS. Treatments are centered on supportive care and include interventions to promote cardiac function and maintain appropriate blood pressure. In severe cases, patients may warrant extra corporeal membrane oxygenation until function recovery.
Reexamining our case, the patient’s decompensation after pericardiocentesis had a broad differential diagnosis including septic shock, worsening acute hypoxemic respiratory failure causing cor pulmonale, and PDS. Blood cultures obtained during the acute decompensation resulted as negative and empiric antibiotics were discontinued after 48 h. Aspergillus galactomannan antigen EIA from the serum resulted as positive. Based on the time course of the patient’s decompensation, rapid recovery, and that the patient’s Aspergillus galactomannan antigen EIA remained positive throughout the patient’s initial recovery, Aspergillus infection was not felt to be the primary driver of the post-pericardiocentesis decompensation. CXR showed no evidence of new pneumonia, but did demonstrate worsening pulmonary edema consistent with PDS. Given these findings in addition to the spontaneous recovery of his RV, PDS is the most likely explanation for our patient’s decompensation.
Our case highlights the diagnostic dilemma regarding the threshold for pursuing pericardiocentesis for pericardial effusion. The initial finding of a small-to-moderate pleural effusion prompted conservative management with diuresis and continued treatment of his underlying CMV infection which was the suspected cause of the pericardial effusion. The patient’s clinical decompensation requiring intubation, ongoing tachycardia, and increased pericardial effusion size prompted our team to intervene with pericardiocentesis. Once the patient developed hemodynamic compromise, the immediate institution of supportive care measures including vasoactive agents and inhaled nitric oxide were critical interventions to maintain adequate hemodynamics. Obtaining a repeat echocardiogram was also critical post-decompensation. It showed the stark contrast from his normal biventricular function earlier in the morning to acute RV failure. This allowed for directed therapies to support the RV until recovery.
The paucity of reported pediatric cases of PDS may be attributed to it being an underrecognized and underreported entity. Further research and awareness are crucial to enhancing our understanding of PDS.
Conclusion
PDS is a rare but serious potential complication of pericardiocentesis. Pediatric cardiologists and intensivists should be aware of this potential complication and consider monitoring patients for at least 48 h after drainage of pericardial effusion. Further investigation into PDS in pediatric patients is needed to guide risk assessment, prevention strategies, and management.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Signed informed consent was obtained from the patient’s mother.
Author Contributions
Dr. Monette Veral: writing (original draft), editing; Dr. Anisha Contractor: conceptualization, writing (original draft), preparation of figures; Dr. Majid Husain: writing (review and editing), preparation of figures; Dr. Ivanna Maxson: writing (review and editing); Dr. Micah Kadden: conceptualization, writing (review and editing).
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
BAL: bronchoalveolar lavage; CMV: cytomegalovirus; CT: computed tomography; CXR: chest X-ray; HFNC: high-flow nasal cannula; HSCT: hematopoietic stem cell transplant; LV: left ventricle; PCR: polymerase chain reaction; PDS: pericardial decompression syndrome; PICU: pediatric intensive care unit; RV: right ventricle; RVSP: right ventricular systolic pressure
| References | ▴Top |
- Amro A, Mansoor K, Amro M, Sobeih A, Suliman M, Okoro K, El-Hamdani R, et al. A comprehensive systemic literature review of pericardial decompression syndrome: often unrecognized and potentially fatal syndrome. Curr Cardiol Rev. 2021;17(1):101-110.
doi pubmed pmc - Prabhakar Y, Goyal A, Khalid N, Sharma N, Nayyar R, Spodick DH, Chhabra L. Pericardial decompression syndrome: a comprehensive review. World J Cardiol. 2019;11(12):282-291.
doi pubmed pmc - Pradhan R, Okabe T, Yoshida K, Angouras DC, DeCaro MV, Marhefka GD. Patient characteristics and predictors of mortality associated with pericardial decompression syndrome: a comprehensive analysis of published cases. Eur Heart J Acute Cardiovasc Care. 2015;4(2):113-120.
doi pubmed - Dosios T, Theakos N, Angouras D, Asimacopoulos P. Risk factors affecting the survival of patients with pericardial effusion submitted to subxiphoid pericardiostomy. Chest. 2003;124(1):242-246.
doi pubmed - Das N, Feingold B. Pericardial decompression syndrome. N Engl J Med. 2023;389(25):e54.
doi pubmed - Imazio M. Pericardial decompression syndrome: a rare but potentially fatal complication of pericardial drainage to be recognized and prevented. Eur Heart J Acute Cardiovasc Care. 2015;4(2):121-123.
doi pubmed - Vandyke WH, Jr., Cure J, Chakko CS, Gheorghiade M. Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med. 1983;309(10):595-596.
doi pubmed - Skalidis EI, Kochiadakis GE, Chrysostomakis SI, Igoumenidis NE, Manios EG, Vardas PE. Effect of pericardial pressure on human coronary circulation. Chest. 2000;117(3):910-912.
doi pubmed - Cerrud-Rodriguez RC, Rashid SMI, Shaqra H, Alkhalil A, Algodi M, Chudow JJ, Garcia MJ, et al. An overlap presentation of pericardial decompression syndrome and stress cardiomyopathy following therapeutic pericardiocentesis. JACC Case Rep. 2020;2(7):1009-1013.
doi pubmed pmc - Wolfe MW, Edelman ER. Transient systolic dysfunction after relief of cardiac tamponade. Ann Intern Med. 1993;119(1):42-44.
doi pubmed - Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, Brucato A, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921-2964.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
