| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Original Article
Volume 13, Number 2, June 2024, pages 41-47
Risk Factors Associated With Early-Selective Bubble Nasal Continuous Positive Airway Pressure and Surfactant Failure in Premature Infants With Respiratory Distress Syndrome
Johanna Marcela Garcia-Valbuenaa, Luis Alfonso Diaz-Martineza, Mario Augusto Rojas-Deviaa, Luis Alfonso Perez-Veraa, b
aSchool of Medicine, Health Science Faculty, Department of Pediatrics, Division of Neonatology, Universidad Industrial de Santander and Hospital Universitario de Santander, Bucaramanga, Colombia
bCorresponding Author: Luis Alfonso Perez-Vera, Neonatal Unit, Pediatrics Department, Hospital Universitario de Santander, Carrera 33 # 28-124, Bucaramanga, Colombia
Manuscript submitted March 12, 2024, accepted April 11, 2024, published online June 23, 2024
Short title: CPAP Failure Risk Factors in Preterm Infants
doi: https://doi.org/10.14740/ijcp519
| Abstract | ▴Top |
Background: The aim of the study was to identify significant risk factors associated with early-selective bubble nasal continuous positive airway pressure (BnCPAP) and surfactant failure in preterm infants with respiratory distress syndrome (RDS).
Methods: This was a retrospective cohort study of 344 premature infants with RDS born at the Hospital Universitario de Santander, Bucaramanga, Colombia, between 2006 and 2015. All infants were required to have clinical evidence of progressive respiratory failure, FiO2 ≥ 0.4, and exposure to BnCPAP and surfactant with the INSURE method as initial management for RDS. The primary outcome was the failure of early-selective BnCPAP and INSURE. The incidence of failure was estimated as a cumulative incidence and incidence density ratio. The strength of association with failure was estimated by binomial regression, Kaplan-Meier survival curves, and Cox’s regression adjusting for birth weight and gestational age (GA).
Results: Early-selective BnCPAP and INSURE failure occurred in 53/344 patients: a cumulative incidence of failure of 14.9% and an incidence density rate of 3.9/1,000 h of BnCPAP exposure. Risk factors associated with failure were: GA < 28 weeks (relative risk (RR) = 2.62, 95% confidence interval (CI): 1.39 - 4.94) and need for resuscitation with positive pressure ventilation (RR = 1.97, 95% CI: 1.09 - 3.54). Additionally, failure was associated with a higher risk of severe intraventricular hemorrhage and death.
Conclusion: GA < 28 weeks and need for positive pressure ventilation during resuscitation were associated with failure of early-selective BnCPAP and INSURE. Early-selective mechanical ventilation and surfactant therapy should be considered in this subgroup of patients.
Keywords: Respiratory distress syndrome; Early-selective surfactant; Continuous positive airway pressure; Preterm infant
| Introduction | ▴Top |
The development of different modes of ventilatory support, exogenous surfactant, and antenatal steroids has significantly decreased mortality and morbidity from respiratory distress syndrome (RDS) [1]. However, exposure to mechanical ventilation has been identified as a significant risk factor associated with pulmonary injury and the development of bronchopulmonary dysplasia (BPD) [2]. Additionally, it has been identified as a risk factor for cerebral palsy and learning disorders in this vulnerable population of infants [3]. Non-invasive modalities of ventilatory like continuous positive airway pressure (CPAP) and early-selective surfactant have been proposed as alternatives to mechanical ventilation [4, 5].
When early-selective CPAP is used as the sole strategy for ventilatory support in the setting of RDS, failure has been documented to be in the range of 36-50% [5-7], and is associated with a substantially higher rate of pneumothorax, a higher risk of death, BPD, and other morbidities [7]. Administration of early-selective exogenous surfactant through transient intubation followed by extubation to bubble nasal CPAP (BnCPAP), also known as the INSURE method, is a validated strategy for the treatment of premature infants with RDS that supports effective and sustains alveolar recruitment, improves lung compliance, shortens the duration of respiratory failure, and substantially decreases the need for mechanical ventilation and the development of associated morbidities [4, 5, 8].
The Neonatal Intensive Care Unit (NICU) at the Hospital Universitario de Santander (UIS) has used early-selective BnCPAP with the INSURE method for the past 10 years as the initial management strategy for preterm infants with RDS [9]. The present study aimed to identify perinatal and neonatal risk factors associated with failure of this strategy in order to anticipate candidates for early-selective intubation and ventilatory support with mechanical ventilation.
| Materials and Methods | ▴Top |
Study design and population
This study included a retrospective cohort of premature infants < 33 weeks of gestation with RDS born at Hospital Universitario de Santander (HUS) between 2006 and 2015. Additional inclusion criteria were: evidence of increased work of breathing during the first hour of life, need for supplemental oxygen with FiO2 ≥ 0.4, administration of early-selective exogenous surfactant (within the first hour of life) with the INSURE method, and ventilatory support with BnCPAP as the initial management of RDS. Exclusion criteria were: the need for intubation and mechanical ventilation as part of initial resuscitation, major congenital abnormalities, and prolonged rupture of membranes > 3 weeks. The Institutional Review Boards of the Universidad Industrial de Santander (UIS) and HUS approved the protocol. This study was conducted in compliance with the ethical standards of the two responsible institutions on human subjects.
Eligible infants were stabilized with the administration of positive end-expiratory pressure (PEEP) of 5 cm H2O using a mask and a T-piece, Neopuff® resuscitator to support lung recruitment and then intubated for the administration of surfactant (Survanta®) 100 mg/kg, followed by the administration of intermittent positive pressure ventilation (PPV) with a T-piece resuscitator (peak inflation pressure (PIP) 20 cm H2O and PEEP of 5 cm H2O) applied for 30 s after the administration of surfactant. Infants were then evaluated for evidence of spontaneous breathing and, if present, were extubated and placed again on T-piece CPAP and PEEP of 5 cm H2O with a facemask for transfer to the NICU. In the NICU, infants were placed on BnCPAP with a pressure of 4 - 6 cm H2O. The BnCPAP depended upon the patient’s clinical evaluation and respiratory failure. Supplemental oxygen was titrated to maintain pulse oximetry saturations between 89% and 96%. Infants who demonstrated worsening respiratory distress and met any of the following CPAP failure criteria: PaCO2 > 65 mm Hg, pH < 7.22, pulse oximetry saturations persistently < 80%, recurrent severe apnea ≥ 20 s, or FiO2 > 0.75 to maintain saturations > 92%) were intubated, placed on mechanical ventilation, and administered additional doses of surfactant according to a pre-established protocol [6].
Relevant clinical data were obtained from the electronic medical record at HUS and registered in pre-designed data collection sheets. Data were transferred to an Excel Microsoft® spreadsheet for subsequent analysis. The primary outcome of the study was a failure on BnCPAP after INSURE. Potential maternal risk factors associated with failure included: diabetes (gestational, type I and type II), hypertensive disorders during pregnancy, urinary tract infections, preterm premature rupture of membranes (PPROM) with prolonged rupture of membranes (> 18 h), and chorioamnionitis (presence of maternal fever associated with one or more of the following: fetal tachycardia (> 160 beats per minute for at least 10 min), maternal leukocytosis > 15,000 in the absence of corticosteroids, evidence of purulent fluid in the cervical canal, or microbiological confirmation of invasion of the amniotic cavity) [10]. Postnatal risk factors included: gestational age (GA), birth weight (BW), sex, mode of delivery, complete or incomplete prenatal steroid administration, Apgar score at 1 and 5 min, need for neonatal resuscitation (PPV + supplemental oxygen, chest compressions, medications), and diagnosis of pneumonia as defined by CDC criteria (radiographic findings of pulmonary consolidation, evidence ventilation or oxygenation impairment and at least three of the following: thermal instability, leukopenia, leukocytosis and/or 10% immature forms, apnea, tachypnea, intercostal retractions, bradycardia, or tachycardia) [11]. Additional long-term outcomes explored to determine their association with failure were: chronic lung disease (need for supplemental oxygen at 28 days of chronological age and on supplemental oxygen at the time of discharge or need for supplemental oxygen at 36 weeks post-menstrual age) associated with clinical or radiographic changes [12], intraventricular hemorrhage (IVH) grade I-IV [13], air leak syndrome (pulmonary interstitial emphysema, pneumothorax, pneumo-pericardium or pneumo-mediastinum) [14], necrotizing enterocolitis (NEC) Bell’s stage ≥ 2A criteria [15], clinically symptomatic confirmed or suspected sepsis [16], and death.
Statistical analysis
Early-selective BnCPAP + INSURE failure was estimated as a cumulative incidence and number of events/1,000 h of CPAP exposure. Nominal and ordinal variables were summarized in the univariate analysis as proportions for each category, while the discrete and continuous variables were summarized as the median and interquartile range (IQR) due to the absence of normal distribution. Two multiple stepwise regression analyses were performed, including all prenatal and postnatal variables significantly associated with failure of early-selective BnCPAP + INSURE in a univariate analysis controlling for BW. First, binomial regression analysis was done to estimate relative risks (RRs) with their corresponding 95% confidence intervals (CIs) for a cumulative incidence of failure. Second, we estimated the survival function free of early-selective BnCPAP and INSURE failure using Kaplan-Meier survival curves and hazard ratio (HR), and their 95% CI were estimated by Cox’s regression for incidence density rate. Finally, we evaluated the strength of the association between early-selective BnCPAP and INSURE failure and secondary outcomes by logistic regression adjusting for BW to produce odds ratios (ORs) and corresponding 95% CI. We evaluated the statistical significance of possible associations using the χ2 test for proportions and log-rank for survival curves using an α < 0.05 as a cut-point.
| Results | ▴Top |
Between 2006 and 2015, 395 premature newborns with < 33 weeks of gestation met eligibility criteria. Fifty-one infants from this cohort were excluded: 30 infants had an Apgar score at 5 min of age ≤ 3, six infants had a major congenital anomaly, and 15 infants required intubation during the first 15 min of life as part of resuscitation. A total of 344 premature newborns were included in the study. INSURE failure occurred in 53 infants (15.4%); 25 failures (47.2%) occurred during the first 24 h of life. The cumulative incidence of INSURE failure was 14.9%, with an incidence density of 3.9 failures for every 1,000 h of BnCPAP exposure. Tables 1, 2, and 3 show the prenatal and postnatal characteristics and risk factors of the cohort categorized by success versus failure of early-selective BnCPAP and INSURE. Only BW and GA, maternal chorioamnionitis, need for resuscitation, pneumonia, and initial PEEP were significantly different between groups. Binomial regression analysis adjusted for BW found two risk factors associated with BnCPAP and INSURE + failure: GA < 28 weeks (RR = 2.62, 95% CI: 1.39 - 4.94) and the need for resuscitative measures with PPV (RR = 1.97, 95% CI: 1.09 - 3.54).
 Click to view | Table 1. INSURE Failure Incidence According to Maternal Prenatal Exposures |
 Click to view | Table 2. Failure Rate of Early-Selective BnCPAP and INSURE According to Year and Neonatal Demographics |
 Click to view | Table 3. BnCPAP and INSURE Failure Incidence According to Postnatal Clinical Characteristics |
Figure 1a shows the survival function free of BnCPAP and INSURE failure showing it occurs between 2.0 and 78.5 h after initiation of BnCPAP. The risk of failure is relatively similar during the first 48 h but increases after this period (Fig. 1b). Cox’s regression identified GA < 28 weeks (HR = 4.02, 95% CI: 1.73 - 9.34), maternal history of chorioamnionitis (HR = 2.60, 95% CI: 1.36 - 4.96), being male (HR = 2.22, 95% CI: 1.22 - 4.05), and an initial PEEP of 6 cm H2O (HR = 2.96, 95% CI: 1.01 - 8.68) as significant risk factors associated with a shorter time interval to failure. These findings are corroborated when analyzing time to BnCPAP failure using Kaplan-Meier curves (Figs. 2-4).
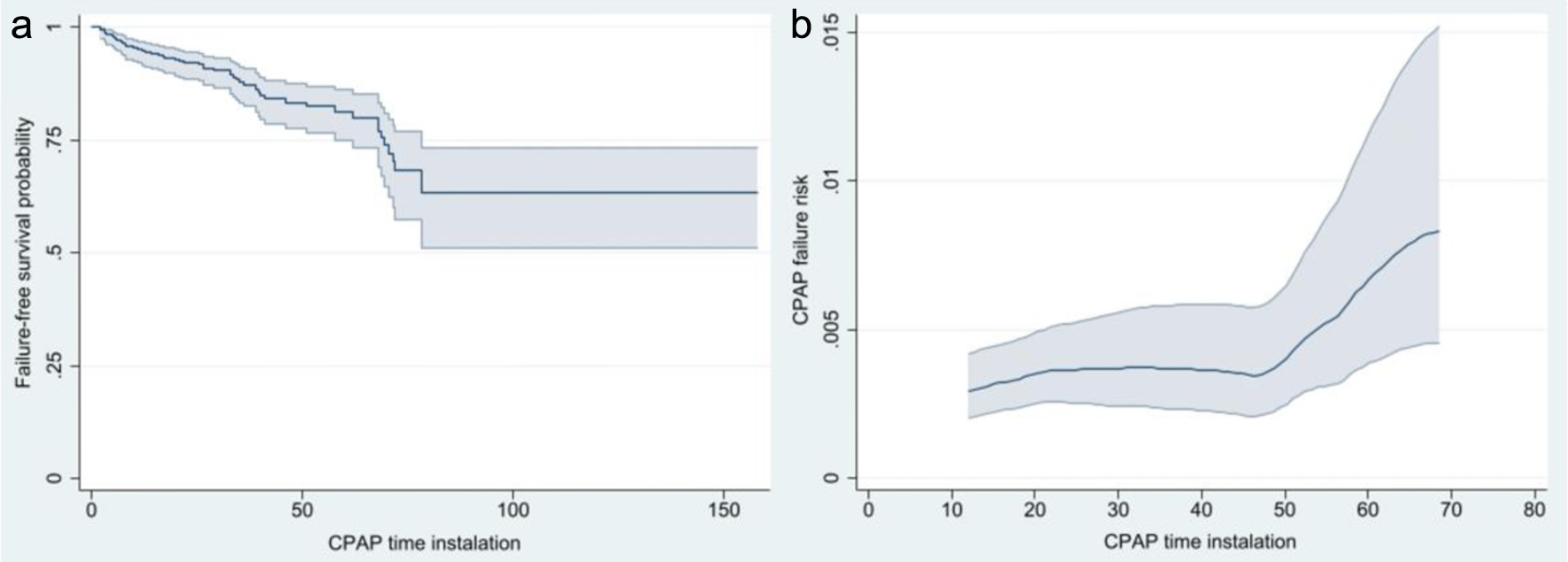 Click for large image | Figure 1. Risk of survival free of CPAP failure (a) and risk of CPAP failure with corresponding 95% confidence intervals (b). CPAP: continuous positive airway pressure. |
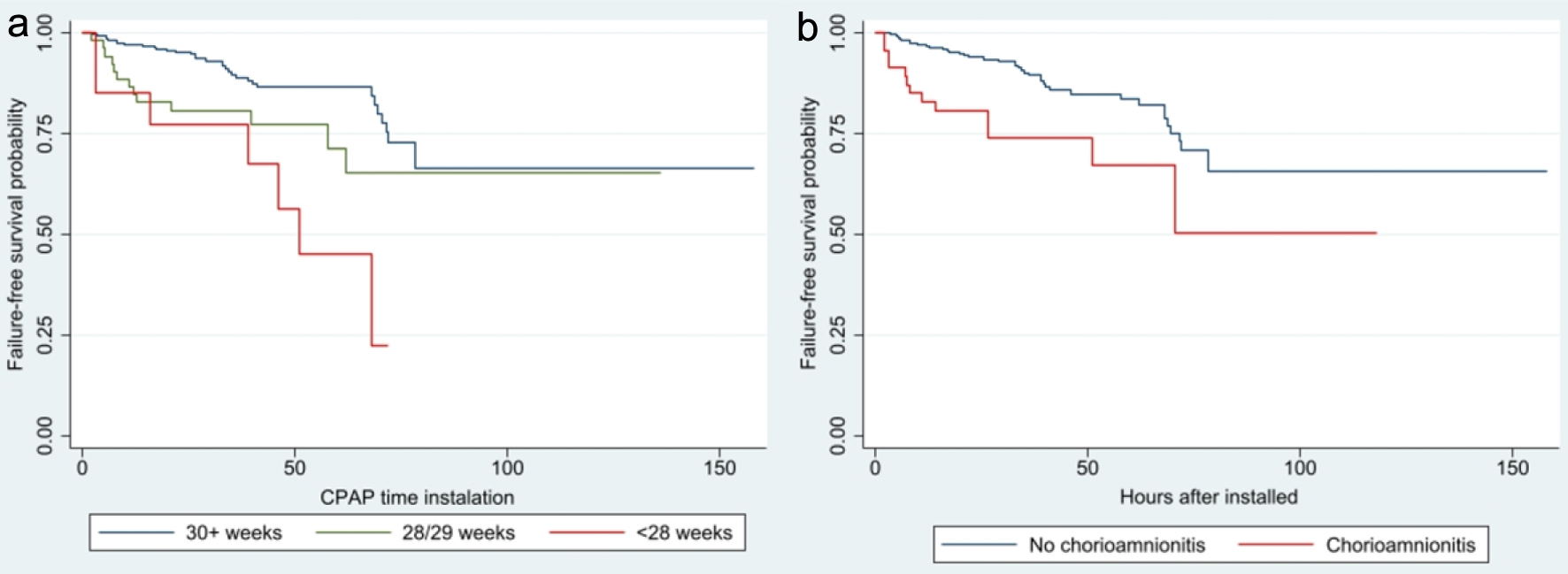 Click for large image | Figure 2. Survival analysis of CPAP failure according to gestational age (a) (P < 0.001) and maternal history of chorioamnionitis (b) (P = 0.002). CPAP: continuous positive airway pressure. |
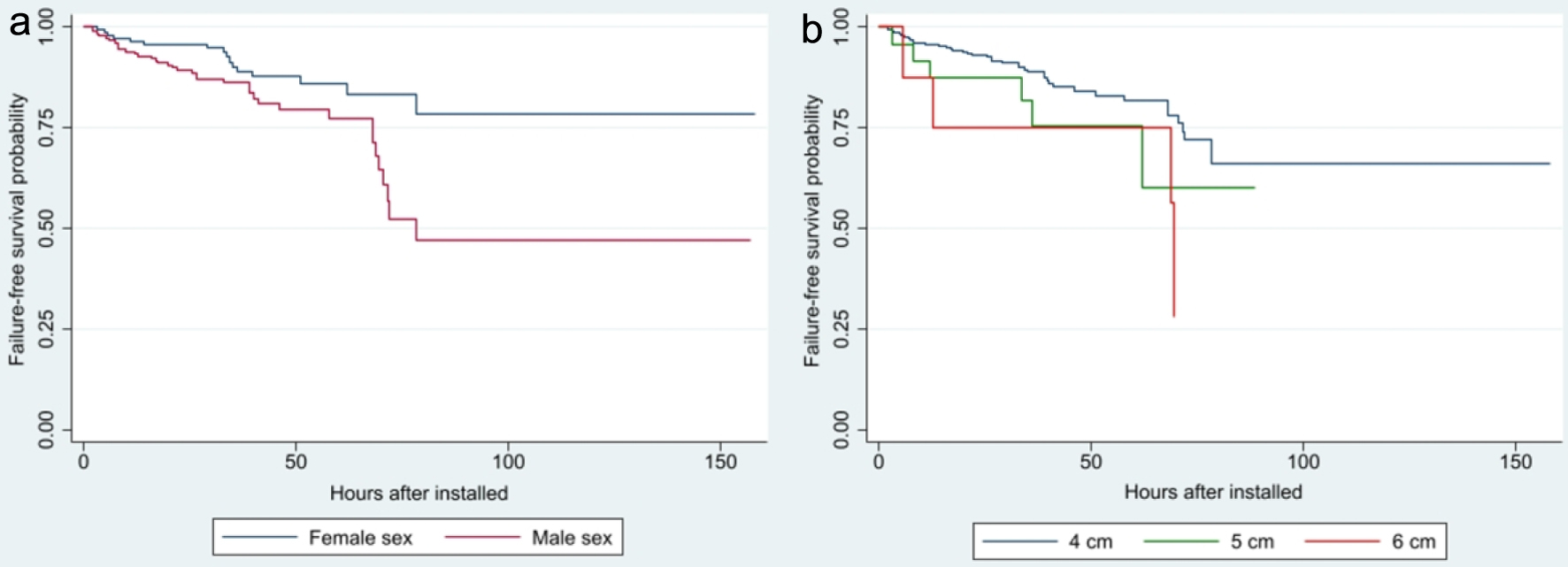 Click for large image | Figure 3. Survival analysis of CPAP failure according to neonatal sex (a) (P = 0.007) and initial PEEP value (b) (P = 0.083). CPAP: continuous positive airway pressure; PEEP: positive end-expiratory pressure. |
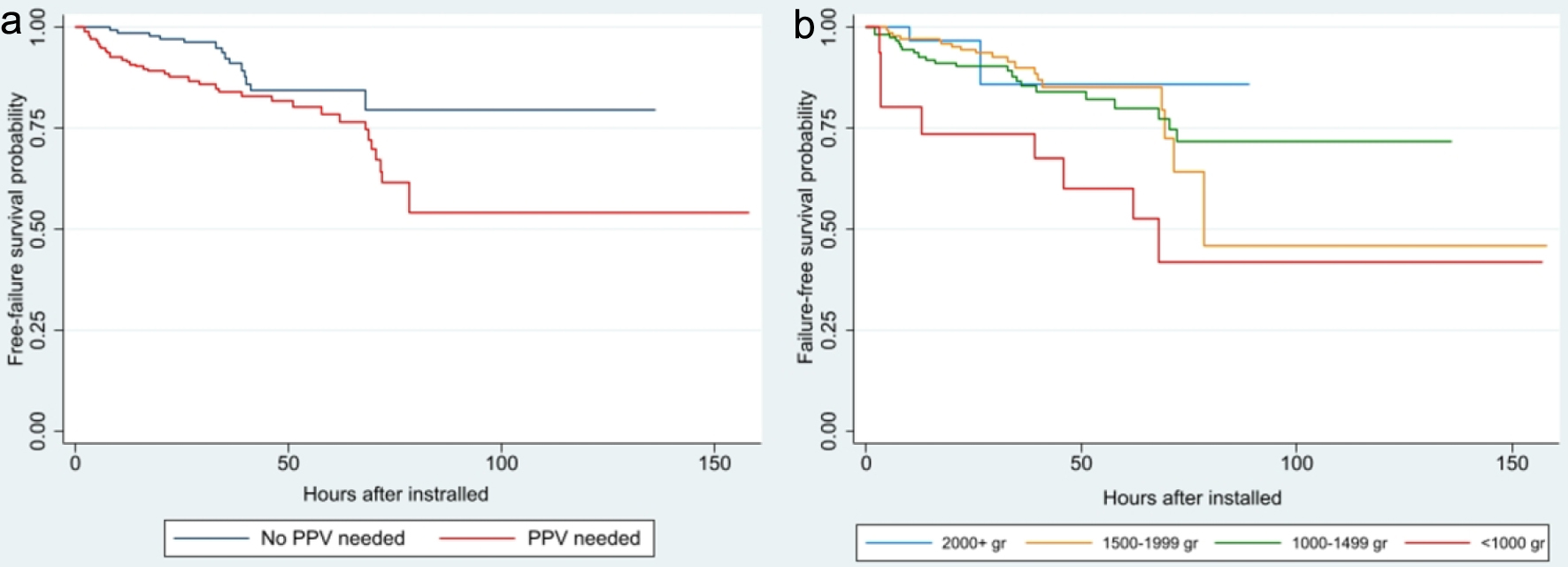 Click for large image | Figure 4. Survival analysis of CPAP failure according to need for resuscitation (a) (P = 0.014) and weight at birth (b) (P = 0.040). CPAP: continuous positive airway pressure; PPV: positive pressure ventilation. |
The incidence of secondary outcomes is shown in Table 4. In addition, INSURE and BnCPAP failure is related to the incidence of severe IVH, even after adjusting for BW (OR = 16.46, 95% CI: 1.74 - 155.46). Newborn infants < 28 weeks of GA have a higher risk of failure (OR = 29.37, 95% CI: 2.53 - 341.12). Finally, the death risk related to BnCPAP and INSURE failure was not significant (RR = 1.91, 95% CI: 0.49 - 4.61), but it is significant among those newborns with a GA of < 28 weeks (RR = 9.89, 95% CI: 2.72 - 35.95).
 Click to view | Table 4. Incidence of Secondary Outcomes According to INSURE Success or Failure |
| Discussion | ▴Top |
In this study, we found BnCPAP and INSURE failure in 14.9% of patients, with 47.2% occurring during the first 24 h after its administration. Risk factors associated with failure were a GA < 28 weeks and the need for resuscitation with PPV. Early failure after the intervention was associated with a lower GA, chorioamnionitis, and being male. Additionally, severe IVH (III and IV) was associated with failure.
These results are similar to those reported by Rojas et al [5], in a randomized controlled study of early-selective BnCPAP and INSURE. Our incidence of failure is significantly lower than reported previously in studies of CPAP without early-selective surfactant [7]. The COIN study reported a failure rate of 46% in newborns between 25 and 28.5 weeks of gestation treated with CPAP without early-selective surfactant [17]. Our rate of pneumothorax (2.0%) was also significantly lower than the 9.1% observed in the COIN study [17]. The lower gestation of the population of preterm infants in the COIN study may partly explain this difference, but the presence of a significantly higher rate of pneumothorax suggests that lack of early-selective surfactant as an explanatory factor [7]. Similarly, the CURPAP study found a failure rate of 33%, with an incidence of pneumothorax of 6.7% in infants < 28 weeks gestation exposed to prophylactic nasal CPAP [18].
Regarding the factors related to CPAP failure, Ammari et al found failure in 24-50% of newborns weighing less than 1,250 g and 750 g, respectively, and identified a shorter GA (< 26 weeks), low BW (< 750 g), and the severity of the RDS as risk factors [6]. In our study, GA was a crucial predicting factor. However, most of the GAs were above 28 weeks, and only four patients were between 24 and 26 weeks. Additionally, all our patients received surfactant before BnCPAP. Cherif et al described 109 patients that received surfactant and CPAP with a 32.1% failure rate, twice ours. Failure in their study was associated with weighting less than 1,000 g, the partial pressure level of CO2, and severe radiographic changes as predictors [8]. They did not take into account clinical parameters like Apgar score at 1 min and the need for resuscitation, but they considered blood gas and radiographic parameters that were not considered in this study because once our patients presented with early progressive RDS, we administered BnCPAP and INSURE, and obtained a chest film [8].
A small study by Koti et al found significant radiographic changes, not receiving antenatal steroids, patent ductus arteriosus, sepsis/pneumonia, and FiO2 ≥ 50% after 20 min on BnCPAP without INSURE as significant risk factors for failure [19]. In our study, antenatal steroids were not associated with failure. Additionally, Rocha et al found failure was associated with the need for FiO2 > 0.3 during resuscitation, a BnCPAP pressure of > 6 cm H2O, and FiO2 > 0.4 in the first 4 h of life [20]. The need for postnatal supplemental oxygen and high CPAP appears to be proxies for surfactant deficiency and severe respiratory distress. However, in our study, the highest proportion of patients received a CPAP of 4 cm H2O after early-selective surfactant avoiding the need for higher CPAP.
Recently, Dargaville et al reported a cohort study that evaluated 19,103 newborns between 25 and 28 weeks, and 29 and 32 weeks that received CPAP without INSURE versus mechanical ventilation with surfactant therapy. CPAP failure was observed in 43% and 21%, respectively and was associated with an increase in the rate of pneumothorax (8.1% and 11% versus 0.36% and 0.37%) [7]. In our study, CPAP failure was lower, and the frequency of pneumothorax was only 3.8% in patients with CPAP failure and 1.7% among those where it was successful supporting the need for administering early-selective surfactant in the management of these patients. Additionally, given that the risk of INSURE failure increased after 48 h of its administration, we consider it necessary to conduct a new study to evaluate the need for a second dose of surfactant.
In summary, GA of < 28 weeks and the need for resuscitative measures with PPV are factors associated with BnCPAP and INSURE failure. Additionally, failure was associated with an increased risk of severe IVH and death in preterm infants < 28 weeks gestation. We recommend including these risk factors when considering the need for intubation and management with mechanical ventilation and surfactant in preterm newborns with evidence of RDS.
Acknowledgments
We thank the administration of the Hospital Universitario de Santander for facilitating the information required to conduct this study. We also thank the Universidad Industrial de Santander with logistic and biostatistical support.
Financial Disclosure
The study was performed with institutional funding from Universidad Industrial de Santander as a graduate thesis.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
JMG-V, LAD-M, MAR-D and LAP-V all designed the study, collected data, run analysis and participated in the writing of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon request.
| References | ▴Top |
- Rojas MA. Equipoise in research and the development of neonatal interventions for the management of respiratory distress syndrome: a historical perspective. Am J Perinatol. 2015;32(10):910-915.
doi pubmed - O’Reilly M, Sozo F, Harding R. Impact of preterm birth and bronchopulmonary dysplasia on the developing lung: long-term consequences for respiratory health. Clin Exp Pharmacol Physiol. 2013;40(11):765-773.
doi pubmed - Aly H. Mechanical ventilation and cerebral palsy. Pediatrics. 2005;115(6):1765-1767.
doi pubmed - Verder H, Bohlin K, Kamper J, Lindwall R, Jonsson B. Nasal CPAP and surfactant for treatment of respiratory distress syndrome and prevention of bronchopulmonary dysplasia. Acta Paediatr. 2009;98(9):1400-1408.
doi pubmed - Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, Charry L, et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics. 2009;123(1):137-142.
doi pubmed - Ammari A, Suri M, Milisavljevic V, Sahni R, Bateman D, Sanocka U, Ruzal-Shapiro C, et al. Variables associated with the early failure of nasal CPAP in very low birth weight infants. J Pediatr. 2005;147(3):341-347.
doi pubmed - Dargaville PA, Gerber A, Johansson S, De Paoli AG, Kamlin CO, Orsini F, Davis PG, et al. Incidence and outcome of CPAP failure in preterm infants. Pediatrics. 2016;138(1):e20153985.
doi pubmed - Cherif A, Hachani C, Khrouf N. Risk factors of the failure of surfactant treatment by transient intubation during nasal continuous positive airway pressure in preterm infants. Am J Perinatol. 2008;25(10):647-652.
doi pubmed - Perez LA, Gonzalez DM, Alvarez KM, Diaz-Martinez LA. [Nasal CPAP versus mechanical ventilation in 28 to 32-week preterm infants with early surfactant administration]. Biomedica. 2014;34(4):612-623.
doi pubmed - Higgins RD, Saade G, Polin RA, Grobman WA, Buhimschi IA, Watterberg K, Silver RM, et al. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol. 2016;127(3):426-436.
doi pubmed pmc - Hooven TA, Polin RA. Pneumonia. Semin Fetal Neonatal Med. 2017;22(4):206-213.
doi pubmed pmc - Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82(4):527-532.
pubmed - Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534.
doi pubmed - Ibrahim CP, Ganesan K, Mann G, Shaw NJ. Causes and management of pulmonary air leak in newborns. Paediatr Child Health. 2009;19:165-170.
- Walsh MC, Kliegman RM, Fanaroff AA. Necrotizing enterocolitis: a practitioner’s perspective. Pediatr Rev. 1988;9(7):219-226.
doi pubmed - Haque KN. Definitions of bloodstream infection in the newborn. Pediatr Crit Care Med. 2005;6(3 Suppl):S45-S49.
doi pubmed - Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, Investigators CT. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358(7):700-708.
doi pubmed - Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, Mosca F, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics. 2010;125(6):e1402-e1409.
doi pubmed - Koti J, Murki S, Gaddam P, Reddy A, Reddy MD. Bubble CPAP for respiratory distress syndrome in preterm infants. Indian Pediatr. 2010;47(2):139-143.
doi pubmed - Rocha G, Flor-de-Lima F, Proenca E, Carvalho C, Quintas C, Martins T, Freitas A, et al. Failure of early nasal continuous positive airway pressure in preterm infants of 26 to 30 weeks gestation. J Perinatol. 2013;33(4):297-301.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
