| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Case Report
Volume 11, Number 2, June 2022, pages 60-65
Salmonella Osteomyelitis in Two Immunocompetent Children
Jelle Nederstigta, c, Navin N. Ramrattana, Lindy J.F. Janssenb
aOrthopedic Surgery, Curacao Medical Center, J. H. J. Hamelbergweg, Willemstad, Curacao
bPediatrics, Curacao Medical Center, J. H. J. Hamelbergweg, Willemstad, Curacao
cCorresponding Author: Jelle Nederstigt, Orthopedic Surgery, Curacao Medical Center, J. H. J. Hamelbergweg, Willemstad, Curacao
Manuscript submitted April 19, 2022, accepted June 9, 2022, published online June 16, 2022
Short title: Salmonella Osteomyelitis in Immunocompetent Children
doi: https://doi.org/10.14740/ijcp487
| Abstract | ▴Top |
Intraosseous infection of Salmonella species typically occurs in long bones in children with hemoglobinopathies, like sickle cell anemia. Osteomyelitis of the pelvis or vertebral body in healthy children is extremely rare. This is especially so when occurring in the same period in the same geographical area. Antibiotic treatment alone can be effective; however, in case of a persistent ongoing infection or foreign body in situ, surgical drainage should be considered. We present two cases of young adolescents, both 15 years old, presenting with Salmonella infection causing osteomyelitis and late hardware infection in Curacao.
Keywords: Osteomyelitis; Salmonella species; Infection of internal fixation devices; Immunocompetent; Child
| Introduction | ▴Top |
Infections with a Salmonella species are common both in the developing and industrialized world and are more prevalent in children [1]. Salmonella spp. is known to be able to cause osteomyelitis, especially in immunocompromised children [2] and children with sickle cell disease [3]. Osteomyelitis with a Salmonella species in immunocompetent children however is rare [4, 5]. Involvement of non-tubular bones is even more unusual [4]. Here we present two cases of immunocompetent adolescent males with osteomyelitis due to a Salmonella spp. in a non-tubular bone. We also review the epidemiology, differential diagnosis, and treatment options. This research was approved by the ethical board of the Curacao Medical Center.
| Case Reports | ▴Top |
Case 1
Presentation
A 15-year-old mixed race (African and Caucasian) boy presented in the outpatient clinic during routine follow-up after scoliosis correction with posterior spondylodesis 4 years before, in which he received preoperative vancomycin prophylaxis and vancomycin and cefepime due to a multilobar pneumonia postoperatively. Wound healing postoperatively had been unremarkable, and he had been without complaints from the postoperative period until the episode currently presented.
His past medical history reported an operation on coarctation of the aorta. He received all vaccinations according to the local vaccination program. He is the oldest of two children from a Caucasian father and African Caribbean mother, neither of them is known with sickle cell trait, nor is he. Before this episode he was a physically active child playing regular sports at school.
Initially, at his first visit presented here, he only complained of mild pain, without fever, no loss of appetite, and no abnormalities upon physical examination. No trauma had occurred. Approximately 4 weeks later, at his second visit, the pain had increased, still without any other signs of infection. Two weeks later, at his third visit, he had developed a fluctuating subcutaneous swelling of approximately 10 cm in diameter in the caudal part of the surgical scar around thoracic vertebra 12 and lumbar vertebra 1 (Fig. 1). He still had no loss of appetite and no neurological deficit. On admission his temperature was 37.4 °C with a heart rate of 87 beats per minute (bpm) and a blood pressure of 102/65 mm Hg. His parents recalled he had gastroenteritis 5 months before from which he recovered in a few days.
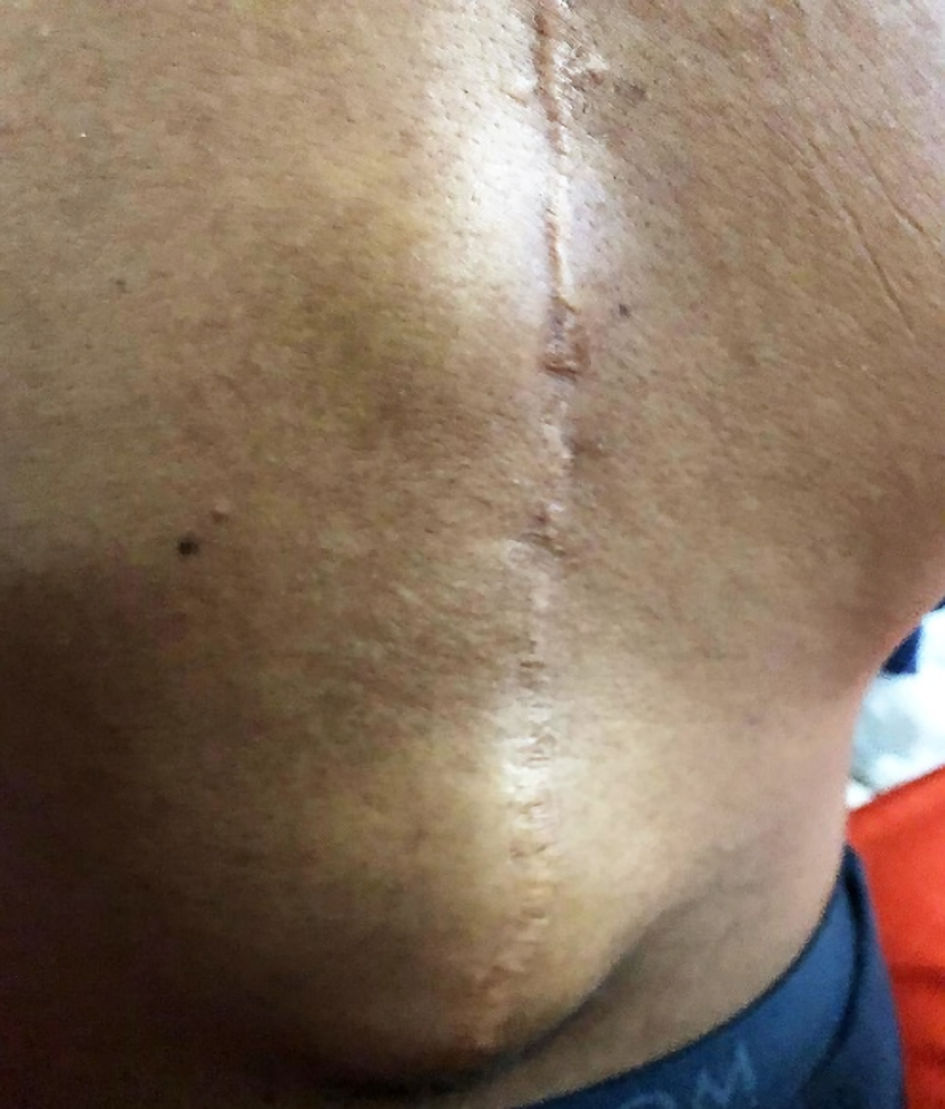 Click for large image | Figure 1. Photo of fluctuating subcutaneous swelling in the caudal part of the surgical scar around thoracic vertebra 12 and lumbar vertebra 1. |
Biochemical findings
Six weeks after first complaints, he had a normocytic anemia (hemoglobin (Hb) 6.4 g/dL, hematocrit (Ht) 0.33, erythrocytes 3.89 × 1012/L, mean corpuscular volume (MCV) 86 fL, mean corpuscular hemoglobin (MCH) 1.6 pg, mean corpuscular hemoglobin concentration (MCHC) 19.2 g/dL, red cell distribution width (RDW) 13.5%), normal thrombocyte count (363 × 109/L) leukocytosis (12.5 × 109/L), neutrophil leukocytosis (9.6 × 109/L), normal sodium (136 mmol/L), normal potassium (4.0 mmol/L), normal renal function (creatinine 71 µmol/L with estimated glomerular filtration rate (eGFR) > 120 mL/min/1.73m2), elevated erythrocyte sedimentation rate (ESR) 112 mm/h and elevated C-reactive protein (117 mg/L). Patient was tested for sickle cell trait, which was negative. His blood type is O, Rh negative without irregular antibodies.
Radiological findings
An ultrasound obtained shortly after his third visit, confirmed a subcutaneous collection measuring 7.7 × 4.8 × 8.1 cm, which then was aspirated and showed a light green, creamy, odorless fluid. A computed tomography (CT) scan was obtained and no lucency around the pedicle screws could be observed (Fig. 2a-c).
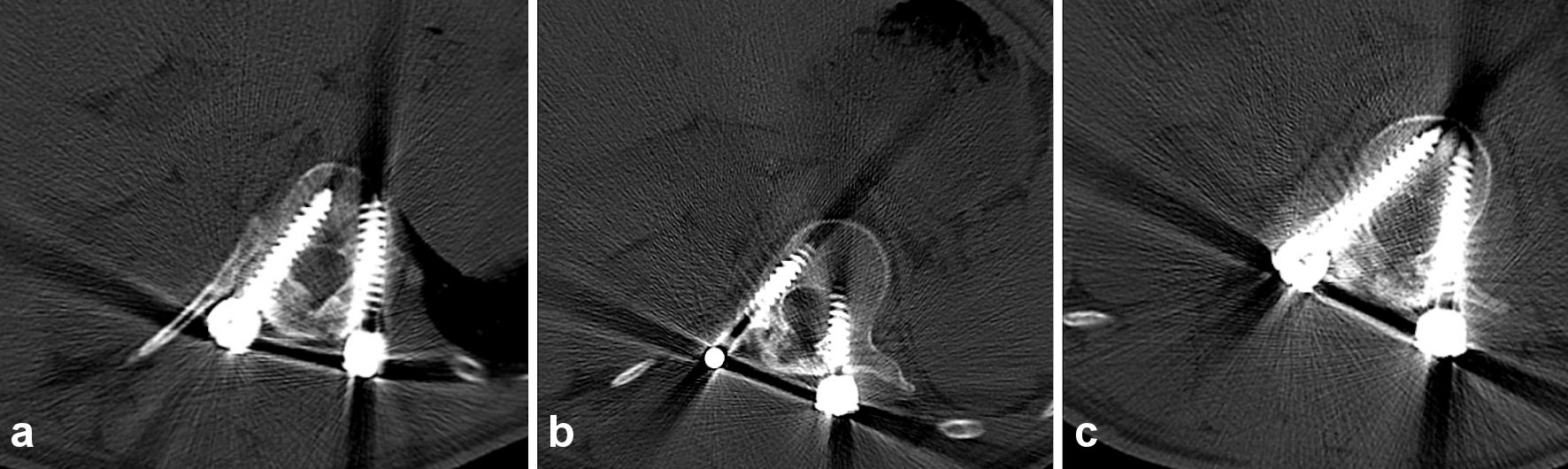 Click for large image | Figure 2. Axial CT of the spine thoracic vertebra 12, bone setting (a), lumbar vertebra 1, bone setting (b), and lumbar vertebra 2, bone setting (c), does not show any lucency around the pedicle screws, subcutaneous collection of fluid is visible dorsal of the spine and screws. CT: computed tomography. |
Surgical procedure
A surgical wash-out was performed. The entire scar was opened, as well as the fascia and all scoliosis hardware were exposed. All pockets of pus were opened and washed out. Deep tissue biopsies were sent for microbiological analysis. A curette was used to clean all surfaces and pulse lavage was used to wash out the entire wound. Swabs soaked with diluted povidone-iodine in Ringer lactate were placed in the wound for several minutes, after which the wound was washed out again with 0.9% NaCl. The wound was then closed.
Microbiological findings
Aerobic and anaerobic blood cultures were not obtained because patient did not meet sepsis criteria according to the systemic inflammatory response syndrome. Salmonella spp. was found in cultures of the biopsied tissue and remained negative for any other bacterial pathogen. No further species determination could be performed. Tuberculosis was not tested, as this is not endemic in Curacao.
Outcome
Postoperatively, the patient received intravenous sulfamethoxazole-trimethoprim three times a day 960 mg as a Staphylococcus aureus infection was expected initially. However, when after 4 days Salmonella spp. was identified instead, 2,000 mg intravenous ceftriaxone was administered bi-daily instead for a total duration of 4 weeks. The maximum dosage was used to eliminate Salmonella spp. without hardware removal. Postoperative pain was treated with four times a day acetaminophen 1,000 mg and three times a day diclofenac 50 mg, both administered by suppository. Four weeks after wash-out, infection parameters had decreased but the ultrasound showed a persisting abscess measuring 4.8 × 2.3 × 17 cm. The patient underwent a second surgical wash-out similar to the first one, and intravenous ceftriaxone administration was continued. Two weeks after this second wash-out, preoperatively obtained tissue cultures were still negative and intravenous ceftriaxone was discontinued and oral administration of ciprofloxacin 750 mg bi-daily was commenced for another 3 months. The patient was followed in our outpatient clinic, where after approximately 2 months of oral antibiotics he did not have any symptoms of infection or pain. At 6 months and 1 year after the second wash-out the patient remained free of symptoms of infection, the C-reactive protein was 6 mg/L.
Case 2
Presentation
A second 15-year-old Caucasian boy presented to the emergency department of our hospital, 6 weeks after case 1, complaining of pain in his right hip, inability to walk because of pain and fever. He had no relevant past medical history or history of trauma. He is a school-going adolescent who plays football in his free time. He is vaccinated according to the local vaccination program. His family history is unremarkable, and neither of his parents nor he is known with sickle cell trait. The pain in his right buttock had started 3 weeks before, after an initial period of high fever. The pain had increased in the 3 days prior to presentation to a point he was not able to ambulate anymore. Physical examination revealed tenderness in the right hip on palpation, the hip was not painful in full range of movement when muscles were relaxed. No local swelling, erythema, or skin lesions were evident upon physical examination. Sensitivity and strength of the right hip, upper and lower leg were all in normal range and did not differ compared to the left side. Knee-jerk reflex and Achilles tendon reflexes were present symmetrically. His body temperature was 38.5 °C, heart rate of 143 bpm, blood pressure of 120/81 mm Hg and saturation of 96% without extra oxygen. He demonstrated no concomitant symptoms. Upon admission patient received four times a day acetaminophen 1,000 mg and three times a day diclofenac 50 mg, both intravenously administered, which was switched to oral administration after 2 days.
Biochemical findings
Laboratory investigations showed Hb 8.5 g/dL, Ht 0.40, erythrocytes 5.34 × 1012/L, MCV 75 fL, MCH 1.6 pg, MCHC 21.1 g/dL, RDW 13.5%), normal thrombocyte count ( 192 × 109/L), leukocytosis (12.4 × 109/L), neutrophil leukocytosis (11.2 × 109/L), lymphocytopenia (0.5 × 109/L), hyponatremia (132 mmol/L), normal potassium (3.9 mmol/L), normal renal function (creatinine 54 µmol/L with eGFR > 120 mL/min/1.73 m2), normal ESR 9 mm/h and elevated C-reactive protein (158 mg/L). Patient was tested for sickle cell trait which was negative. His blood type is AB, Rh negative without irregular antibodies.
Radiological findings
A CT scan of the pelvis was performed and showed an osteolytic lesion in the right sacrum. There was an asymmetry of the gluteal muscles where the soft tissues on the right side seemed enlarged (Fig. 3). A magnetic resonance imaging (MRI) scan was performed to rule out malignancy (Fig. 4). This showed edema in the right sacrum with a small fluid collection adjacent to the SI joint. An ultrasound of the abdomen was performed to exclude signs of gastrointestinal bacterial translocation, which was negative.
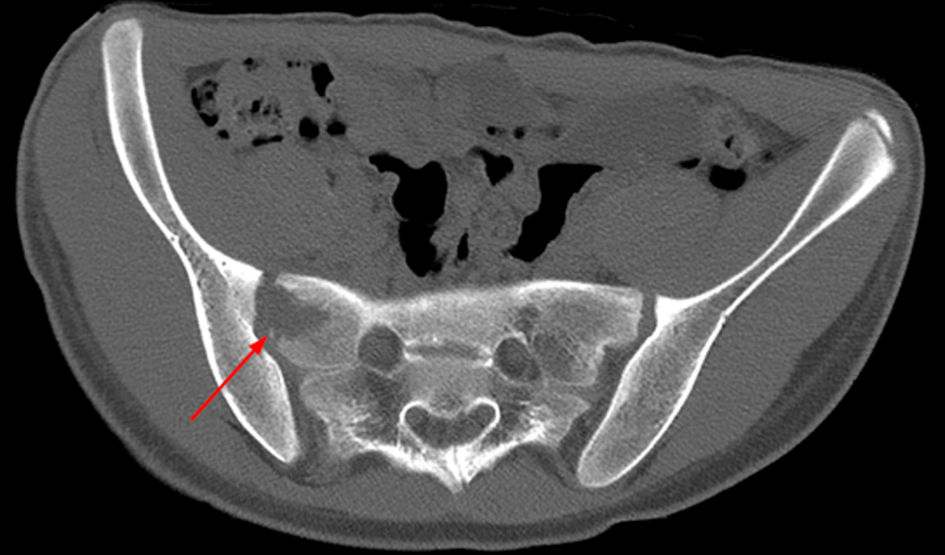 Click for large image | Figure 3. Axial CT of the pelvis, bone setting, showing an osteolytic lesion in the right sacrum (arrow). CT: computed tomography. |
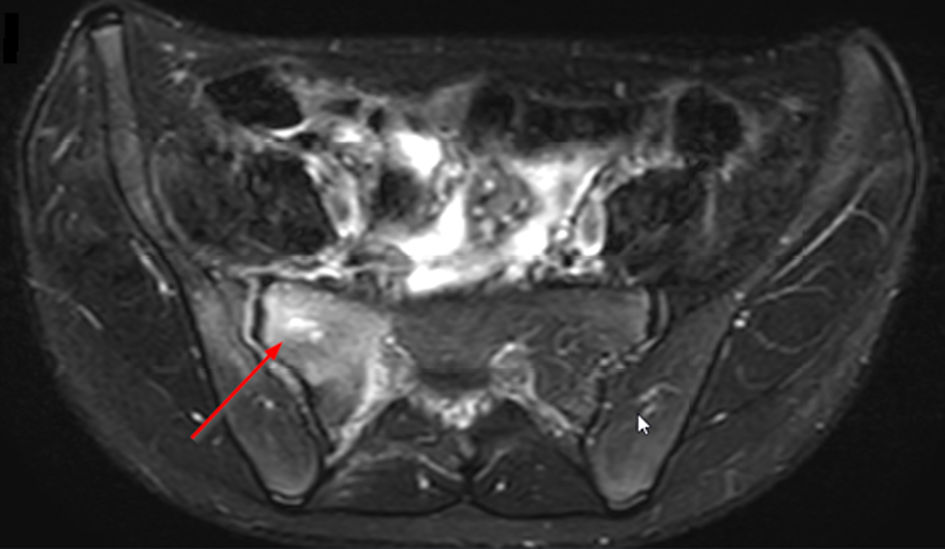 Click for large image | Figure 4. Axial T2-weighted SPAIR sequence MRI of the pelvis showing osteolytic lesion in the right sacrum (arrow). MRI: magnetic resonance imaging. |
Microbiological findings
Aerobic and anaerobic blood cultures were obtained which showed a Salmonella spp and remained negative for any other bacterial pathogen. Tuberculosis was not tested, as this is not endemic in Curacao.
Antibiotic treatment
After isolation of Salmonella spp., 2,000 mg of ceftriaxone per day was intravenously administered for 14 days. After this period, 750 mg of ciprofloxacin was orally administered, twice a day for 3 months.
Outcome
Following the initial 14 days of intravenous antibiotic treatment, the patient was discharged. After 1 month he was able to mobilize without any pain and without symptoms of infection. After 6 months and 1 year follow-up there were no symptoms of infection present, C-reactive protein remained below 4.0 mg/L and the boy was discharged from follow-up.
Differential diagnosis
The main categories of differential diagnosis to be considered in cases like the ones presented should be infectious diagnosis, oncological diagnoses, and traumatic diagnosis.
Traumatic injury can cause pain and swelling of the bone and joints. Careful history taking should guide the clinician towards this diagnosis and orthopedic consultation may be indicated.
If osteomyelitis is suspected, depending on the geographical prevalence, analyses for Mycobacterium tuberculosis should be considered. As tuberculosis is not endemic in Curacao and our patients both had a positive culture for Salmonella spp., this was not tested in our cases. Acute osteomyelitis needs to be distinguished from septic arthritis, cellulitis, and soft tissue infections. Apart from infectious osteomyelitis, the clinician should consider chronic nonbacterial osteomyelitis (CNO), an (auto-)inflammatory bone disease of unknown origin that primarily affects children and adolescents. Clinical presentation and severity of CNO may vary significantly between individual patients, covering a wide spectrum with asymptomatic or mild inflammation of single bone at one end, and chronic recurrent multifocal, and sometimes bone destruction causing osteomyelitis at the other end [6]. It is a diagnosis of exclusion.
In sickle cell patients, a vaso-occlusive crisis can mimic osteomyelitis. It can be very challenging to differentiate between these two. Even though osteomyelitis is 50 times less common than a vaso-occlusive crisis, their clinical features are very similar. Both often occur in long bones and present with pain, swelling and fever. Elevated inflammatory parameters can be found in both conditions but tend to be more distinctly elevated in osteomyelitis. The “gold standard” to discriminate osteomyelitis from a vaso-occlusive crisis is a positive culture, however blood cultures can remain negative. MRI can be a good adjunct to distinguish as it is highly sensitive (97%) for diagnosing osteomyelitis as well as highly specific (92%) ruling it out [3, 7]. We recommend obtaining an MRI after 3 days of onset of complaints and performing a biopsy when blood cultures remain negative, and MRI does not distinguish between the two or is not available.
As in our both cases, sickle cell disease was excluded with a high-performance liquid chromatography.
Pain and swelling could occur due to a malignancy. Age, radiological appearance, and localization are the main factors to affect the likelihood of different oncological diagnoses. During adolescence, a malignancy (e.g., Ewing sarcoma or osteosarcoma) has to be ruled out, especially when the lesion shows osteolytic characteristics like those presented in case 2. Ewing sarcoma can show a large soft tissue mass without calcifications or bone matrix in the tissue. A multilayered so-called “onion-skin” periostitis is typical [4]. Approximately 25% of cases occur in the pelvis and 8% in the spine. Pelvic localization seems to be associated with higher mortality and morbidity as the tumor can readily grow into the pelvic cavity. The proximity of internal organs and neurovascular bundles combined with a lack of anatomic barriers make local control of pelvic sarcomas difficult [8]. Sacral localization, however, is thought to have a better outcome compared to other pelvic sites as it is more superficial and closer to the sacral nerves which will lead to symptoms in an earlier stage [9]. Osteosarcoma occurs in 4-10% of the pelvis, where it shows cortical bone destruction and periostic reactions. Osteosarcoma in the pelvis is associated with delay in diagnosis and poor outcome [10].
Taking age and localization and epidemiology into account, only the diagnosis osteoid osteoma, osteoblastoma and aneurysmal bone cyst deserve consideration. The former two are benign bone-forming tumors and can occur in the thoracic and lumbar spine. The latter is also benign, but osteolytic, and occurs in the vertebral column in 3-30% of cases, especially in the lumbar spine. Patients have a similar presentation (back pain that is dull and difficult to localize). Typically, pain in osteoid osteoma occurs mostly at night and responds well to nonsteroidal anti-inflammatory drugs. This is not the case in osteoblastoma. A CT scan typically shows a nidus less than 2 cm in case of an osteoid osteoma and the lack thereof in an osteoblastoma. Aneurysmal bone cysts may show fluid-fluid levels in an osteolytic, expansile cavity on CT and MRI [10, 11].
| Discussion | ▴Top |
This case report describes a Salmonella osteomyelitis in two healthy young boys in a non-tubular bone. Even though Salmonella is known to be able to cause osteomyelitis, it usually occurs in predisposed patients and in tubular bones. The finding of a Salmonella as a pathogen for osteomyelitis in non-predisposed patients and also in a non-tubular bone is extremely rare. No other reports have been found in literature.
The causative organism that is highlighted in this case report is the Salmonella species, gram-negative non-sporeforming rods that are classified in the Enterobacteriaceae family. They are usually transmitted through contaminated water and food and can cause enterocolitis, typhoid fever, bacteremia, or local infection in a chronic state. Salmonella spp. is an encapsulated organism which prevents the organism from phagocytosis and destruction of immune cells. It replicates in the cell. Salmonella spp., can, although rarely so (< 1%), cause osteomyelitis [12]. The three strains to be known to cause osteomyelitis are Salmonella typhi, Salmonella typhimurium and Salmonella enteritidis [13].
An intraosseous infection by Salmonella spp. occurs more frequently in sickle cell patients [12]. Intravascular sickling causes capillary occlusion of the gastrointestinal tract, which permits organisms to cross the gastrointestinal wall into the bloodstream [14]. Other predisposing conditions include iatrogenic or hereditary immunosuppression, connective tissue disorders, previous surgery and hematological neoplasms [5, 15].
In healthy children, Salmonella osteomyelitis is rare and accounts for only 0.5% of all osteomyelitis cases. Involvement of the spine is in only one in four of these, with only three cases of pelvic involvement earlier reported [5].
Osteomyelitis in children has an incidence varying from 1 to 13 per 100,000 in developed countries to 200 per 100,000 in developing countries [15]. There is a higher prevalence in children younger than 6 years old. Boys are affected twice as often as girls, most likely because they are more physically active, which is thought to predispose them to repeated microtrauma [7]. The majority of pediatric osteomyelitis cases are secondary to hematogenous spread and typically occur in tubular bones, because of a rich vascularity, low flow, and a high concentration of hematopoietic marrow in the metaphysis [7]. Involvement of other bones, such as the pelvis (6.3-20%) and spine (1-2%) is unusual, especially in immunocompetent children [2, 4, 16]. Early detection and treatment are important as osteomyelitis can cause deformities, growth arrest, chronic infection and in case of the spine spread to the spinal cord can cause serious neurological complications [15, 16]. The most common causative organism of osteomyelitis in children is Staphylococcus aureus, followed by Kingella kingae, streptococcal and gram-negative organisms such as Salmonella spp. [2, 4, 15, 16]. Animal studies suggest that osteolysis is caused by the immune response rather than the pathogen in bacterial bone infections [17].
There are only a few case reports of osteomyelitis with Salmonella spp. in the spine in healthy children. Therefore, clear characteristics of these patients cannot be given. When we look at all vertebral osteomyelitis within children, it seems to be uncommon and only accounts for 1-2% of children with osteomyelitis. Children are usually over 7 years of age and most often the lumbar or thoracic region is affected [18].
In case of pediatric osteomyelitis of the pelvis, the ilium is most commonly affected followed by the ischium, pubis and acetabulum. Children often present with pain in the hip, thigh, or abdomen, often causing them to limp or refuse to walk. Local signs of infection can be present. In Salmonella infections, the presentation is often acute with fever, chills, elevated infectious parameters, and severe pain; however, as our case 1 showed, this can be absent. The delay from onset until presentation varies from 1 to 2 weeks and has caused permanent disability in 3.4% of cases [5].
To prevent this disability, treatment has to be started prompt after diagnosis, ideally after bone and blood cultures have been drawn. There are no globally accepted standards for the treatment of Salmonella osteomyelitis. The most commonly used antibiotics are third-generation cephalosporins and fluoroquinolones. Ciprofloxacin is particularly suitable as it has good tissue penetration and the ability to penetrate macrophages and eliminate intracellular bacteria. It has shown good efficacy in treating bone infections [19]. Radical surgical debridement and/or percutaneous radiologic drainage must be considered when there is a poor response to antibiotics in all children with Salmonella osteomyelitis [19]. This is different for infection of internal fixation devices with Salmonella. If the implant is stable, debridement and drainage should be conducted in combination with long-term antimicrobial therapy. Treatment must be aggressive to prevent chronic infection and loosening of the device. If necessary, debridement should be repeated. It should be considered to remove or replace the device if feasible. Preferred antimicrobial treatment of hardware infection with Salmonella spp. is intravenous ceftriaxone for 2 - 4 weeks, followed by, when susceptible to quinolones, ciprofloxacin 750 mg bi-daily. If the device is not removed, the minimum total antimicrobial treatment duration is 3 months [20]. In both our cases good outcome was achieved with this therapy, without having to remove the hardware in case 1 and no permanent disability or chronic infection in both cases.
Conclusions
We present this case report to show that osteomyelitis due to a Salmonella species is rare in immunocompetent children but does occur. Clinicians should rule out associated sickle cell disease and immunodeficiency. To prevent permanent disability among children, clinicians should be aware that, even though rare, a Salmonella osteomyelitis is not impossible in immunocompetent children. Furthermore, Salmonella osteomyelitis should be treated aggressively, especially when there is a hardware infection. Cultures should be obtained before starting antibiotics, usually third-generation cephalosporins and fluoroquinolones.
Acknowledgments
We thank Annette Stemerding for providing useful information in the microbiology field. We thank the parents for permitting us to use the patients’ data.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare they have no competing interests.
Informed Consent
Written informed consent was obtained from the patients for publication of this case report, the images, and all information contained.
Author Contributions
JN drafted the manuscript, carried out the literature research, and prepared the illustrations. NR helped draft the manuscript, performed the surgical wash-outs, follow the patients in the outpatient department and did the final proofreading of the manuscript. LJ helped draft the manuscript. All the authors read and approved the final manuscript
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CT: computed tomography; MRI: magnetic resonance imaging; CNO: chronic nonbacterial osteomyelitis
| References | ▴Top |
- Sanchez-Vargas FM, Abu-El-Haija MA, Gomez-Duarte OG. Salmonella infections: an update on epidemiology, management, and prevention. Travel Med Infect Dis. 2011;9(6):263-277.
doi pubmed - Klein JD, Leach KA. Pediatric pelvic osteomyelitis. Clin Pediatr (Phila). 2007;46(9):787-790.
doi pubmed - Al Farii H, Zhou S, Albers A. Management of osteomyelitis in sickle cell disease: review article. J Am Acad Orthop Surg Glob Res Rev. 2020;4(9):e20.00002-10.
doi pubmed - van Schuppen J, van Doorn MM, van Rijn RR. Childhood osteomyelitis: imaging characteristics. Insights Imaging. 2012;3(5):519-533.
doi pubmed - Canessa C, Trapani S, Campanacci D, Chiappini E, Maglione M, Resti M. Salmonella pelvic osteomyelitis in an immunocompetent child. BMJ Case Rep. 2011;2011:bcr0220113831.
doi pubmed - Zhao Y, Ferguson PJ. Chronic nonbacterial osteomyelitis and chronic recurrent multifocal osteomyelitis in children. Pediatr Clin North Am. 2018;65(4):783-800.
doi pubmed - Jaramillo D, Dormans JP, Delgado J, Laor T, St Geme JW, 3rd. Hematogenous osteomyelitis in infants and children: imaging of a changing disease. Radiology. 2017;283(3):629-643.
doi pubmed - Fan H, Guo Z, Fu J, Li X, Li J, Wang Z. Surgical management of pelvic Ewing's sarcoma in children and adolescents. Oncol Lett. 2017;14(4):3917-3926.
doi pubmed - Saccomanni B. Osteoid osteoma and osteoblastoma of the spine: a review of the literature. Curr Rev Musculoskelet Med. 2009;2(1):65-67.
doi pubmed - Fuchs B, Hoekzema N, Larson DR, Inwards CY, Sim FH. Osteosarcoma of the pelvis: outcome analysis of surgical treatment. Clin Orthop Relat Res. 2009;467(2):510-518.
doi pubmed - Galgano MA, Goulart CR, Iwenofu H, Chin LS, Lavelle W, Mendel E. Osteoblastomas of the spine: a comprehensive review. Neurosurg Focus. 2016;41(2):E4.
doi pubmed - Saturveithan C, Arieff A, Premganesh G, Sivapathasundaram N. Salmonella osteomyelitis in a one year old child without sickle cell disease: a case report. Malays Orthop J. 2014;8(2):52-54.
doi pubmed - Fontalis A, Hughes K, Nguyen MP, Williamson M, Yeo A, Lui D, Gelfer Y. The challenge of differentiating vaso-occlusive crises from osteomyelitis in children with sickle cell disease and bone pain: A 15-year retrospective review. J Child Orthop. 2019;13(1):33-39.
doi pubmed - Anand AJ, Glatt AE. Salmonella osteomyelitis and arthritis in sickle cell disease. Semin Arthritis Rheum. 1994;24(3):211-221.
doi - Iliadis AD, Ramachandran M. Paediatric bone and joint infection. EFORT Open Rev. 2017;2(1):7-12.
doi pubmed - Palmowski Y, Burger J, Kienzle A, Trampuz A. Antibiotic treatment of postoperative spinal implant infections. J Spine Surg. 2020;6(4):785-792.
doi pubmed - Luthje FL, Jensen LK, Jensen HE, Skovgaard K. The inflammatory response to bone infection - a review based on animal models and human patients. APMIS. 2020;128(4):275-286.
doi pubmed - Tyagi R. Spinal infections in children: A review. J Orthop. 2016;13(4):254-258.
doi pubmed - McAnearney S, McCall D. Salmonella osteomyelitis. Ulster Med J. 2015;84(3):171-172.
- Trampuz A, Zimmerli W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury. 2006;37(Suppl 2):S59-S66.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
