| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Case Report
Volume 11, Number 2, June 2022, pages 51-55
Neonatal Blues: Cyanosis and Failure to Thrive in a Newborn
Jennifer E. Hollanda, Scott B. Yeagerb, c, Richard H. Flyerd, Jonathan N. Flyerb, c, e
aThe Robert Larner, M.D. College of Medicine at The University of Vermont, Burlington, VT 05401, USA
bDepartment of Pediatrics, The Robert Larner, M.D. College of Medicine at The University of Vermont, Burlington, VT 05401, USA
cDivision of Pediatric Cardiology, The University of Vermont Children’s Hospital, Burlington, VT 05401, USA
dIndependent Researcher, Shelburne, VT, USA
eCorresponding Author: Jonathan N. Flyer, Department of Pediatrics, The University of Vermont Children’s Hospital, Burlington, VT 05401, USA
Manuscript submitted March 31, 2022, accepted April 15, 2022, published online June 16, 2022
Short title: Cyanosis and Failure to Thrive in a Newborn
doi: https://doi.org/10.14740/ijcp486
| Abstract | ▴Top |
Congenital heart disease is a spectrum of structural anatomic defects, and clinical manifestations arise from a combination of fixed anatomy and dynamic physiologic processes. We describe a newborn with acyanotic congenital heart disease who rapidly developed hypoxemia, cyanosis, respiratory distress, and failure to thrive. Less than 2 weeks after birth, an unusual constellation of cardiac anatomy (a large ventricular septal defect, double orifice mitral valve, and supramitral ring) cumulatively yielded cyanotic physiology. The acute clinical change prompted immediate anti-congestive therapy and urgent cardiothoracic surgery. This case is the first report of successful neonatal cardiac surgery for this cyanotic constellation of defects, which are independently classified as acyanotic structural defects. It is an important reminder that newborns with multiple intracardiac lesions may behave unpredictably throughout the neonatal transition period, and the cardiac differential diagnosis need not be strictly tied to the more common cyanotic anatomy (i.e., limited to one of the “classic five T’s” and single ventricle).
Keywords: Cardiology; General pediatrics; Newborn
| Introduction | ▴Top |
Structural heart disease is the most common type of congenital defect, affecting nearly 1% of newborns. The general pediatric approach to congenital heart disease (CHD) often stratifies cardiac abnormalities into acyanotic and cyanotic subgroups. Common defects generally categorized as acyanotic include atrial and ventricular septal defects, aortic and pulmonary valvar stenosis, as well as structural and functional abnormalities of the atrioventricular valves. Typical lesions usually considered cyanotic CHD include tetralogy of Fallot, transposition of the great arteries, truncus arteriosus, total anomalous pulmonary venous connections, tricuspid atresia (the “classic five T’s”) and various types of single ventricle.
Cyanosis due to CHD is a manifestation of the cardiovascular physiology, which remains heavily dependent on the cardiac anatomy. However, dynamic features of the circulation will affect intracardiac mixing and blood flow, sometimes resulting in cyanosis from lesions not typically thought to yield cyanotic CHD. This report presents a newborn with three independently acyanotic structural heart defects that cumulatively resulted in cyanotic disease, and is the first case of successful neonatal cardiac surgical treatment for this rare anatomic constellation of CHD.
| Case Report | ▴Top |
Investigations
A 31-year-old female, gravida 3, para 2, was referred for fetal cardiac evaluation at 22 weeks gestation due to a maternal family history of hypoplastic left heart syndrome (nephew) and transposition of the great arteries (uncle). The general prenatal history was notable for preeclampsia without severe features. Fetal echocardiography demonstrated a large ventricular septal defect (VSD), normal valves, chamber dimensions and morphologies, and unobstructed ventricular outflows.
The infant was born at 39 weeks via spontaneous vaginal delivery, with a birthweight of 3.5 kg, and Apgar score of 8 and 9. The initial postnatal cardiopulmonary exam was normal, and the peripheral capillary oxygen saturation (SpO2) was 99%. The general newborn and neurologic exams were normal. An electrocardiogram (ECG) demonstrated a sinus rhythm, northwest ventricular axis, and normal precordial voltages. Transthoracic echocardiography, performed in the newborn nursery, confirmed a large unrestrictive VSD and a patent foramen ovale with low velocity left-to-right flow. The mitral valve also appeared abnormal with mildly thickened leaflets and reduced separation, consistent with mild stenosis. The infant was discharged home with pediatric cardiology outpatient follow-up in addition to routine newborn care.
At 10 days of age, the infant presented to the cardiology clinic in respiratory distress, with tachypnea, subcostal retractions, and hypoxemia (SpO2 91%). There were new cardiac exam findings of a prominent systolic murmur and diastolic rumble, and a new finding of left atrial enlargement on ECG. Repeat echocardiography demonstrated the actual diagnosis.
Diagnosis
Repeat echocardiography at 10 days of life revealed persistent, accelerated left-to-right atrial shunting via the foramen ovale with a mean estimated gradient of 16 mm Hg, indicative of left atrial hypertension (Fig. 1). Additionally, there was worsening mitral stenosis with a mean estimated inflow gradient of 10 mm Hg, bidirectional VSD flow, and preserved ventricular function. Careful review of the mitral valve increased suspicion for more serious anatomic abnormalities, including a double orifice mitral valve (DOMV) and supramitral ring (SMR).
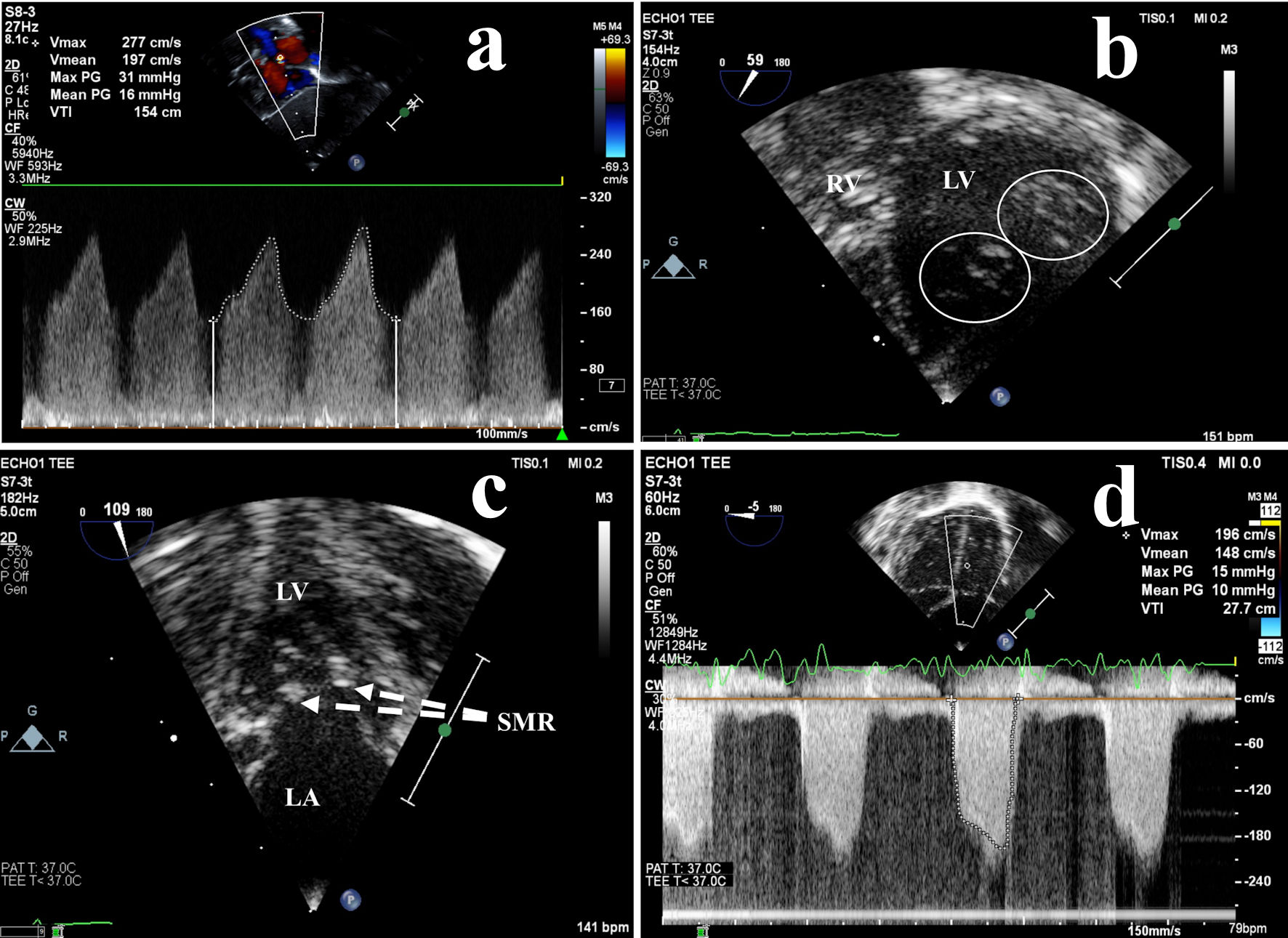 Click for large image | Figure 1. Preoperative echocardiography: left atrial hypertension due to obstructive mitral valve abnormalities. (a) High velocity left-to-right atrial shunt across the foramen ovale measured by transthoracic continuous wave Doppler, indicative of LA hypertension. Transesophageal echocardiography more clearly demonstrated (b) a double orifice mitral valve (circles) and (c) a supramitral ring (dashed lines), resulting in (d) moderate mitral valve inflow obstruction (stenosis). LA: left atrium; LV: left ventricle; PG: peak gradient; SMR: supramitral ring; RV: right ventricle; V: velocity. |
Other possible etiologies for newborn cyanosis with respiratory distress were considered, including pulmonary, hematologic, and infectious disorders. Primary pulmonary causes that impair oxygenation, such as alveolar hypoplasia or surfactant deficiencies, generally present at birth with an abnormal SpO2. Structural airway abnormalities, such as severe trachea- or bronchomalacia, or congenital cystic airway lesions, would likely demonstrate pulmonary auscultatory findings in addition to hypoxemia at birth. A diagnosis of heritable hemoglobinopathies also seemed remote given a negative family history and the absence of cyanosis in more immediate neonatal period [1]. While infections causing pneumonia, bronchiolitis, or even sepsis are important diagnostic considerations, particularly in the neonatal period, our patient had a normal temperature, blood pressure, and there were no respiratory symptoms in family members. Additionally, the new abnormal findings on ECG and echocardiography made these other non-cardiac elements of the differential diagnosis far less likely.
Treatment
Oral furosemide was started, and elective VSD surgery was planned for 4 - 6 weeks of age. Preoperative regional management discussions concluded that cardiac catheterization was not necessary. Over the next week the infant demonstrated increasing lethargy, prolonged feeding, weight loss (20 g), persistent pallor, tachypnea, and declining SpO2 (83-85%).
Shortly after 3 weeks of age, the infant underwent uncomplicated surgical VSD closure with autologous pericardium. Direct surgical inspection of the mitral valve identified a membranous ridge of supramitral tissue extending between two orifices, which was consistent with final diagnosis of both DOMV and SMR, in addition to the VSD. The membrane was resected, while the double orifice was not addressed due to concern for creating significant regurgitation. A minor ridge of subaortic accessory tissue was also identified and resected. Postoperative echocardiography showed mild mitral regurgitation, no aortic regurgitation, no residual VSD, and good ventricular function.
Follow-up and outcomes
The postoperative recovery was uncomplicated, and by 2 weeks following surgery the infant was clinically well, with normal respiratory effort, a normal SpO2, and a 400 g weight gain. Over the following 3 years, the respiratory status, SpO2, weight gain, and development remained normal, and there was no recurrence of the SMR or progression of mitral valve obstruction due to the DOMV.
| Discussion | ▴Top |
We report a case of three independent, typically acyanotic intracardiac lesions (VSD, DOMV, and SMR) that collectively produced cyanosis and failure to thrive (FTT) by 2 weeks of age. Noninvasive cardiac imaging identified the VSD with bidirectional flow, as well as abnormal mitral valve function that suggested evolving obstruction from both the DOMV and the SMR. Early neonatal cardiac surgery eliminated the ventricular shunt and relieved the effective mitral stenosis, correcting the mechanisms for respiratory distress and hypoxemia, and allowing the newborn to thrive.
VSDs are abnormal communications between the left and right ventricle, often associated with additional cardiac defects, and serve as the substrate for high volume and pressure intracardiac shunts [2]. A DOMV is a fibrous accessory tissue bridge that partially or completely creates two mitral orifices [3]. A SMR is a thin membrane or thick fibrous ridge partially or completely encircling the mitral orifice, and may adhere to the mitral leaflets [4]. Both DOMV and SMR are abnormalities that may create functional mitral stenosis, impairing diastolic filling and increasing left atrial pressure.
While VSDs are common, DOMV and SMR are rare. A study of 46 children with DOMV (median age 2.4 years) showed that partial atrioventricular septal defects were the most commonly associated cardiac lesion [5]. Thirteen patients presented with symptoms of congestive heart failure and two patients presented with FTT. Five patients had accompanying traditional cyanotic cardiac lesions, including transposition of the great arteries, truncus arteriosus, tricuspid atresia, and Ebstein’s anomaly [5].
In one single-center retrospective review of clinical and echocardiographic data of patients with SMR (n = 57), the median age at diagnosis was 1.8 years. Thirty-two patients underwent surgical SMR resection. Of those 32, 22 patients had prior non-mitral valve surgery, including VSD closure in four and coarctation repair in 16 [6]. In a different single-center review of 27 pediatric patients who underwent surgery for SMR as the primary cause for significant mitral stenosis, the mean age at the time of surgery was 3.9 years. Other associated cardiac defects were common, most often VSDs (52%) and variants of left-sided obstruction, including coarctation (45%) [7]. The SMR was surgically resected first, and other cardiac defects were repaired if determined to be clinically significant. The overall 20-year survival rate was 82%, with age (< 1 year) and Shone’s anomaly identified as significant risk factors for mortality. In both SMR studies, the reported ages at time of diagnosis or surgery suggested FTT was uncommon.
We hypothesize that the DOMV and SMR in our patient combined to create left ventricular inflow obstruction (Fig. 2), raising left atrial and pulmonary venous pressures, and resulting in pulmonary vascular congestion. The elevated pulmonary venous pressure would also inhibit the usual decline of pulmonary vascular resistance, leading to an increasing element of right-to-left flow across the VSD. It is also likely that the left-to-right component of the VSD shunting led to increased left atrial and mitral valve flow, further raising left atrial pressure.
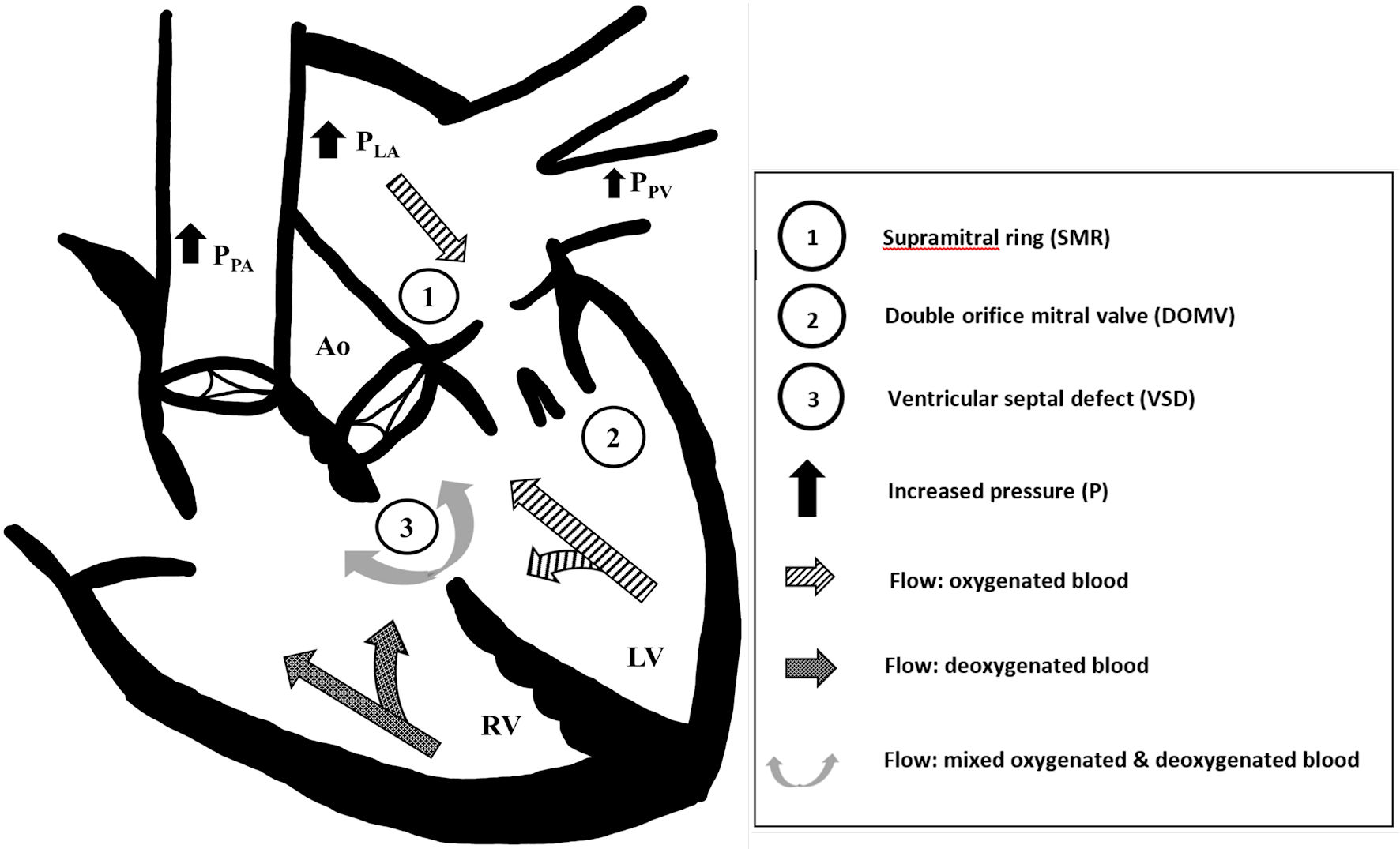 Click for large image | Figure 2. Constellation of acyanotic congenital heart disease leading to cyanotic physiology. We suspect that the (1) supramitral ring and (2) double orifice mitral valve obstructed left ventricular inflow, which increased left atrial (PLA) and pulmonary venous pressures (PPV), and caused bidirectional flow across the (3) ventricular septal defect (VSD). The left-to-right VSD flow further exacerbated left atrial and pulmonary venous pressures. Systemic desaturation was due to increasing elements of right-to-left VSD flow and pulmonary vascular congestion. Ao: aorta; LV: left ventricle; PLA: left atrium pressure; PPA: pulmonary artery pressure; PPV: pulmonary vein pressure; RV: right ventricle. |
We believe that this infant is the first reported neonate with a VSD, DOMV, and SMR to undergo successful surgical repair. A prior case of a child diagnosed at 4 years of age was complicated by severe pulmonary hypertension, leading to early postoperative mortality [8]. We are unaware of other cases with these three independently acyanotic lesions cumulatively resulting in cyanosis and heart failure during early infancy.
Although fetal echocardiography has decreased the incidence of undiagnosed critical CHD at birth [9, 10], this case highlights its limitations. While large VSDs are often imaged prenatally, many more subtle anatomic details, such as the mitral valve findings in this case, are beyond the diagnostic capabilities of current technology. In addition to fetal echocardiography, future prenatal assessment for families with a significant history of CHD, as described in this case, may also include testing for increasingly numerous genetic markers [11] associated with a variety of syndromes and CHD.
Learning points
For the pediatric trainee, this case highlights that CHD is a spectrum of structural anatomic defects, and clinical manifestations arise from a combination of fixed anatomy and dynamic physiologic processes. While newborns with large VSDs will often develop signs of heart failure, dramatic symptoms in the first 2 weeks of life are unusual, and marked desaturation is not typical. This underscores the critical clinical lesson that when the course of a child with CHD differs from usual expectations, prompt and comprehensive cardiology re-evaluation is warranted. Finally, it serves as a reminder that when faced with a cyanotic child due to CHD, the cyanosis need not be strictly tied to a more common anatomic diagnosis (i.e., limited to one of the “classic five T’s” and single ventricle), and patients with multiple intracardiac lesions may behave unpredictably throughout the neonatal transition period.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Family provided written informed consent for publication of case report.
Author Contributions
JH contributed to conceptualization, research, drafting, figure design, and review. SY assisted in conceptualization and review. RF assisted in drafting and review. JF provided medical care, conceptualization, research, drafting, figure design, and review. All authors approved the final manuscript as submitted.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
CHD: congenital heart disease; DOMV: double orifice mitral valve; FTT: failure to thrive; SMR: supramitral ring; SpO2: peripheral capillary oxygen saturation; VSD: ventricular septal defect
| References | ▴Top |
- Crowley MA, Mollan TL, Abdulmalik OY, Butler AD, Goodwin EF, Sarkar A, Stolle CA, et al. A hemoglobin variant associated with neonatal cyanosis and anemia. N Engl J Med. 2011;364(19):1837-1843.
doi pubmed - Cohen M, Lopez L. Ventricular septal defects. In: Allen HD, Shaddy R, Penny D, Feltes T, F C, eds. Moss & Adams' heart disease in infants, children, and adolescents, including the fetus and young adult, 9th ed. Wolters Kluwer; 2016. p. 783-802.
- Trowitzsch E, Bano-Rodrigo A, Burger BM, Colan SD, Sanders SP. Two-dimensional echocardiographic findings in double orifice mitral valve. J Am Coll Cardiol. 1985;6(2):383-387.
doi - Toscano A, Pasquini L, Iacobelli R, Di Donato RM, Raimondi F, Carotti A, Di Ciommo V, et al. Congenital supravalvar mitral ring: an underestimated anomaly. J Thorac Cardiovasc Surg. 2009;137(3):538-542.
doi pubmed - Zalzstein E, Hamilton R, Zucker N, Levitas A, Gross GJ. Presentation, natural history, and outcome in children and adolescents with double orifice mitral valve. Am J Cardiol. 2004;93(8):1067-1069.
doi pubmed - Schidlow DN, Zaidi A, Gauvreau K, Emani SM, Geva T. Echocardiographic characteristics of annulo-leaflet mitral ring. J Am Soc Echocardiogr. 2015;28(5):541-548.
doi pubmed - Brown JW, Ruzmetov M, Rodefeld MD, Turrentine MW. Surgical strategies and outcomes in patients with supra-annular mitral ring: a single-institution experience. Eur J Cardiothorac Surg. 2010;38(5):556-560.
doi pubmed - Datt V, Khurana P, Aggarwal S, Mishra S, Sujith CN, Virmani S. Perioperative management of a patient with double orifice mitral valve with supramitral ring with subaortic membrane with ventricular septal defect and severe pulmonary hypertension: Report of a rare case. Ann Card Anaesth. 2019;22(2):215-220.
doi pubmed - Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129(21):2183-2242.
doi pubmed - van Velzen CL, Clur SA, Rijlaarsdam ME, Bax CJ, Pajkrt E, Heymans MW, Bekker MN, et al. Prenatal detection of congenital heart disease—results of a national screening programme. BJOG. 2016;123(3):400-407.
doi pubmed - Richards AA, Garg V. Genetics of congenital heart disease. Curr Cardiol Rev. 2010;6(2):91-97.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
