| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Case Report
Volume 11, Number 2, June 2022, pages 45-50
Acute Submandibular Sialadenitis in a 9-Month-Old Infant: A Case Report
Edi Hartoyoa, c, Ira Devi Alamsyah Guntorob, Yulia Margarethb
aTropical Infection Division, Department of Pediatrics, Ulin General Hospital Banjarmasin, South Kalimantan, Indonesia
bDepartment of Pediatrics, Ulin General Hospital Banjarmasin, South Kalimantan, Indonesia
cCorresponding Author: Edi Hartoyo, Tropical Infection Division, Department of Pediatrics, Ulin General Hospital Banjarmasin, South Borneo 70233, Indonesia
Manuscript submitted March 31, 2022, accepted May 2, 2022, published online June 2, 2022
Short title: Acute Submandibular Sialadenitis in an Infant
doi: https://doi.org/10.14740/ijcp484
| Abstract | ▴Top |
In the pediatric population, sialadenitis accounts for nearly 10% of all salivary gland diseases. In this case, the patient is a 9-month-old boy who had acute sialadenitis accompanied by systemic symptoms which made the patient’s condition weak and required hospitalization. Transmission of bacteria from the oral cavity to the salivary ducts and salivary glands (retrograde bacterial contamination of the oral flora) and decreased saliva production due to lack of fluid intake due to cough and cold were suspected as risk factors in this patient. The patient came to the hospital with complaints of swelling of the left neck under the left lower jaw since 10 days before being admitted to the hospital with fever and malaise, accompanied by nausea, vomiting and decreased appetite. Laboratory examination revealed leukocytosis with increased levels of C-reactive protein. Submandibular ultrasound examination revealed multiple bilateral submandibular lymph nodes, especially the left. The results of blood culture examination did not reveal the growth of aerobic bacteria. Fine-needle aspiration examination of left submandibular lymph node biopsy revealed non-specific chronic lymphadenitis. The patient was diagnosed with acute submandibular sialadenitis caused by bacterial infection and received therapy such as hydration, empirical broad-spectrum intravenous antibiotics, and symptomatic treatment.
Keywords: Acute sialadenitis; Pediatrics; Submandibular
| Introduction | ▴Top |
Sialadenitis is an inflammation of the salivary glands. The frequency of submandibular sialadenitis is not clear, and in the pediatric population, there is an estimated 10% of all cases of sialadenitis of the major salivary glands [1]. There is no predilection for race, age, and gender [2].
Bacterial or viral infections can cause sialadenitis by direct inoculation of the ductal epithelium or by ascending oral infection. Infection may also occur retrogradely from septic foci in the oral cavity. In dehydrated patients, reduced salivary flow can cause normal oral flora to enter the salivary glands and cause infection [1].
In this case, the patient had acute sialadenitis accompanied by systemic symptoms such as fever and malaise, accompanied by nausea, vomiting and decreased appetite, which made the patient’s condition weak and required hospitalization. Risk factors that may be related to the cause of patient’s sialadenitis are transmission of bacteria from the oral cavity to the salivary ducts and salivary glands (bacterial contamination of retrograde oral flora) and decreased saliva production due to lack of fluid intake due to coughing and colds. The authors report this case because of the limited literature reviewing sialadenitis with systemic symptoms in which the patient may fall into a systemic condition that makes the infection of sialadenitis more severe.
| Case Report | ▴Top |
A 9-month-old boy was brought by his parents to the Emergency Room in Ulin Hospital, Banjarmasin, South Kalimantan with complaints of swelling of the left neck under the left lower jaw since 10 days before being admitted to the hospital. At first, the swelling was the size of a marble, but according to his parents, it had gotten bigger in the last 4 days. There is no redness (Fig. 1). The child cried when the lump was touched. The patient also had a fever since 10 days before admission to the hospital, fever subsided with anti-pyretic drugs, the temperature was not measured, and the child shivered but did not have seizures. The patient also had a cough 10 days before admission to the hospital, dry cough, no additional breath sounds, did not worsen in the morning/night, and was not accompanied by shortness of breath. In addition, the patient also had a runny nose since 10 days before entering the hospital with clear secretions. Two days before admission to the hospital, the patient vomited two times, did not spray (volume of about 30 - 50 mL each time he vomited, filled with food and milk, without vomiting blood, green or black). Loss of appetite occurred 2 days before admission to the hospital, the patient only ate 1 - 2 times a day as much as half the usual portion and only wanted to breastfed, and did not want to drink formula milk. For the patient, there were no history of same illness or hospitalization before, no history of neck swelling before, no history of allergy or asthma, no history of malignancy, no blood disorder or autoimmune disease, and no history of recent surgery or dental work. The patient was born to a mother with a gestational age of 38 - 39 weeks at term, the birth weight was 3,800 g, the birth length was 52 cm, and the head circumference was not clear. Patient immediately cried after birth (Apgar score was 7-8-9), with no history of active resuscitation and no history of prolonged oxygenation.
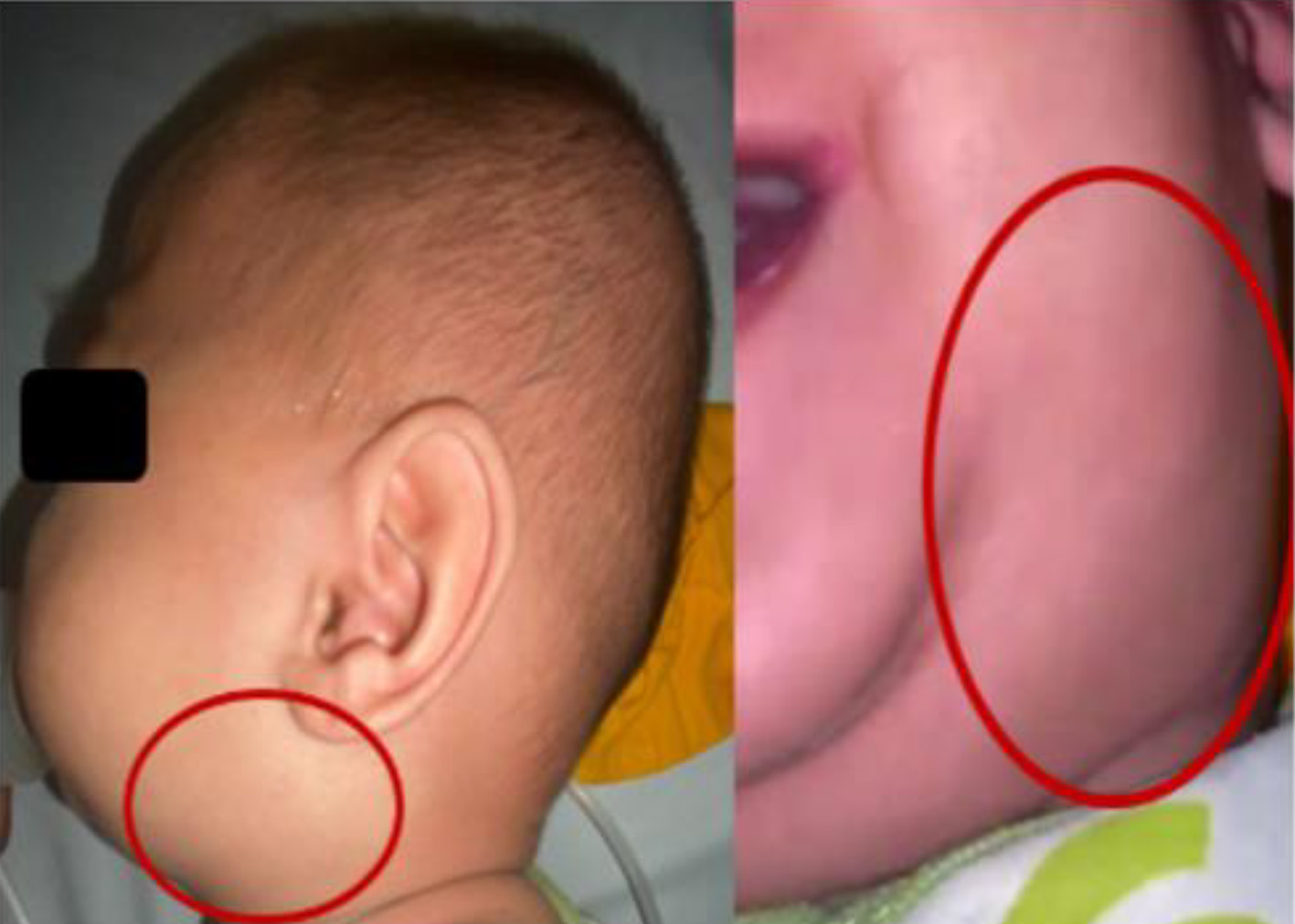 Click for large image | Figure 1. A 2-cm mass at left submandibular region, soft, mobile smooth surface. |
The history of immunization of the patient was bacillus Calmette-Guerin (BCG) (1), hepatitis B (2), diphtheria, tetanus, pertussis (DTP) (3), polio (1), hemophilus influenzae type b (Hib) (1), and measles-rubella (MR) (0), with the conclusion of incomplete immunization (Indonesian Ministry of Health). The patient lives with his parents and his siblings. Their house is far from mining area, dumpster or industrial area. His father is an asphalt paver worker. His mother is a housewife, who sometimes helps grandma to sell fried snack. His grandma sells fried snack several times a week while carrying the patient. His father is a smoker, sometimes smoke at home and outside. There are no mosquito coils usage, and no pets at home. They use boiled tap water for daily needs, drinking and cooking. They use gas stove for cooking. There is no usage of pesticide or other chemical agent regularly in house. There is no family history of the same complaints (neck swelling and sore throat) in the last 2 weeks. Family member did not have complains of cough, runny nose, fever, sore throat, anosmia, shortness of breath, and diarrhea. There is no history of known neighbor infected with tuberculosis. All of them always wear cloth mask outside, and routinely wash their hands and feet every time they get home. The social and living environments of the patient show low socioeconomic status and high-risk infection at home.
On physical examination, the patient was compos mentis, moderately ill, with temperature of 38.6 °C, pulse of 126/min, blood pressure of 90/50 mm Hg (P50-90), respiration rate of 36/min, saturation of 96% in room air, and capillary refill time (CRT) of 2 s. Pain score (visual analog scale) was 3, weight was 8.7 kg, body length was 73 cm, and ideal body weight was 9 kg. The nutritional status of the patient is normal weight, normal stature, and well-nourished.
Physical examination of the head showed normocephaly, no facial edema, no dysmorphic face, no old face, flat open fontanelle 1.5 × 1.5 cm, pale conjunctiva in the eyes, no icteric sclera, isochor pupil 3 mm/3 mm, normal pupillary reflex, normal eye movements, no sunken eyes, and no edema palpebra. In the neck, there was a 2-cm mass at left submandibular region, soft, mobile smooth surface, and minimal tenderness pain. In the ENT, there was no nasal discharge, no nasal flare, hyperemia of pharynx, tonsils (T1/T1), no detritus, no pseudomembranous, and no discharge from both ears. In the mouth, there was no dirty or whitish tongue, no oral thrush, no subgingival bleeding, and no gum swelling. Stensen’s duct in the parotid gland was difficult to evaluate because the patient was uncooperative.
In the heart, sound I-II was normal, and there was no murmur and no gallop. In the thorax at the inspection, there was no wasted ribs and no retraction, symmetrical vocal fremitus at palpation, no axilla lymph nodes enlargement, sonor at both lungs at percussion, no decreased breath sounds at auscultation, no rhonchi, no wheezing, no prolonged expiration, and no stridor. Abdomen was not distended, soft, with normal abdominal sound, no organomegaly, no shifting dullness, no ascites, no palpable mass, and no inguinal lymph nodes enlargement. Extremities were warm, but pale, CRT was less than 2 s, and there was no edema, no crazy pavement dermatosis, no pitting edema, no petechiae, and no hematoma. There was a BCG scar. In the genitalia, there were testis, no swelling, no pain, no redness, and no phimosis.
In the neurological examination, meningeal sign, Brudzinski I, Brudzinski II, and Kernig were negative. Motoric strength was five in all extremities. Sensoria was normal. Physiological reflex (biceps, triceps, knee, and ankle) was normal. Pathological reflexes of Babinski, Chaddock, Oppenheim, Hoffman, and Tromner were negative. There were no spastic, no clonus, and no atrophy on extremity muscle. Cranial nerve (CN) I was hard to evaluate, CN II was afferent. Pupil reflex was normal. CN III, IV, and VI were normal. CN V was sensory. V1, V2, and V3 were hard to evaluate. CN VII showed symmetrical face. CN VIII was hard to evaluate. CN IX/X showed no hoarseness, no dysphagia, and no deviation of uvula. CN XI was hard to evaluate. CN XII showed no tongue deviation and no fasciculation.
The first day laboratory examination results (Table 1) showed leukocytosis of 23,400/µL (neutrophil 40.3% and lymphocyte 47.3%) and C-reactive protein (CRP) of 119.2 mg/L. The patient was diagnosed with acute submandibular sialadenitis caused by bacterial infection and received IV fluid hydration therapy D5 1/4 normal saline (NS) maintenance 800 mL/24 h, ampicillin sulbactam 3 × 450 mg intravenously (dose 150 mg/kgBW/day) and gentamicin 1 × 70 mg intravenously (dose 7.5 mg/kgBW/day), as well as antipyretic paracetamol syrup 100 mg (10 mg/kgBW/time) if fever.
 Click to view | Table 1. Laboratory Results for First Admission on January 24, 2022 |
The patient’s condition for the first 3 days of treatment still had fever (highest temperature 38.5 °C), cough, runny nose and left submandibular swelling. On the fourth day of treatment, the patient had no fever, had a rare cough and minimal runny nose, and reduced swelling of the left neck. Submandibular ultrasound examination (Fig. 2) on the seventh day of treatment revealed multiple bilateral submandibular lymph node enlargement, especially left (left: about 2.07 × 1.78 cm; right: about 1.86 × 0.79 cm). Laboratory evaluation (Table 2) on the seventh day of treatment showed leukocytosis of 15,400/µL (neutrophil 40.7% and lymphocyte 44.7%) and CRP of 25.8 mg/L. The patient was found to be anemic with hemoglobin of 9 g/L (January 24, 2022) and peripheral blood smear examination was performed (Table 3) with normochromic normocytic anemia, leukocytosis, lymphocytosis, atypical lymphocyte results. The results of blood culture examination showed no growth of aerobic bacteria. Examination of fine-needle aspiration biopsy (FNAB) of the left submandibular lymph node on the ninth day of treatment revealed reddish aspirate macroscopically; microscopically, the smear consisted of lymphocytes, centrocytes, and centroblasts (no specific process or malignant tumor cells were seen) with the conclusion of non-specific chronic lymphadenitis.
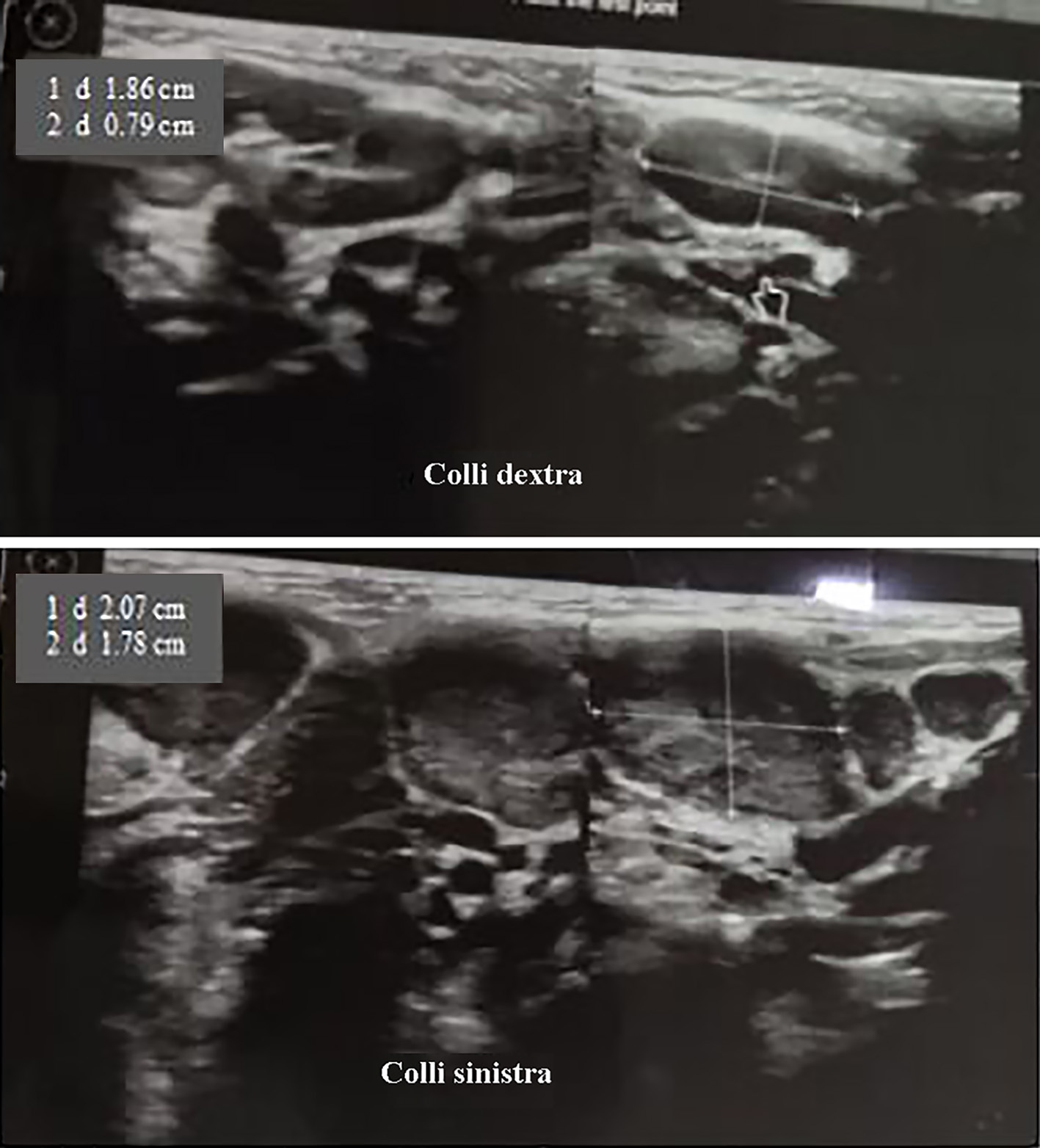 Click for large image | Figure 2. Submandible ultrasonography (January 29, 2022). There are multiple enlargements of bilateral submandible lymph nodes, especially left (left: about 2.07 × 1.78 cm, right: about 1.86 × 0.79 cm). |
 Click to view | Table 2. Laboratory Results of Evaluation of Complete Blood Count on Day 6 (January 29, 2022) |
 Click to view | Table 3. Peripheral Blood Smear on January 24, 2022 |
The patient was allowed to go home after receiving 7 days of intravenous antibiotics and receiving antibiotics co-amoxiclav forte syrup 3 × 6 mL (dose of 100 mg/kgBW/day) for outpatient treatment for 5 days. After 7 days of polyclinic control, the patient came home from the hospital, and there was no swelling in the left neck.
| Discussion | ▴Top |
In this case report, the authors report a case of acute sialadenitis with systemic symptoms which was treated comprehensively so as to prevent the worsening of the condition.
Clinically, acute submandibular sialadenitis is distinguished from parotitis by the site of swelling and discharge of pus. Sialadenitis is an inflammation or infection of the submandibular salivary glands caused by infectious and non-infectious factors. In the United States, the overall incidence is 2 - 8/100,000 people, with the subject neonates to old age [3].
Risk factors associated with infectious sialadenitis are decreased salivary production and decreased salivary flow [3]. Causes of decreased salivary production include autoimmune disorders (primary biliary cirrhosis, sarcoidosis, and Sjogren’s syndrome), dehydration (neonates, premature infants, and the elderly), and iatrogenic causes such as surgery and recent anesthesia, bowel rest, fasting, drugs, radiation therapy, radioactive iodine, and kidney failure. Conditions associated with decreased salivary flow include cystic fibrosis, ductal strictures, sialolithiasis, tumors causing ductal obstruction, iatrogenic injury, immunosuppression, and dental infections [3-5]. In addition, conditions that are at risk for the occurrence of infectious sialadenitis include immunocompromised status, liver failure, congestive heart failure, diabetes mellitus, hypothyroidism, malnutrition, oral infections, oral cavity neoplasms, human immunodeficiency virus, depression, psychiatric disorders, anorexia nervosa, bulimia, hyperuricemia, hyperlipoproteinemia, lead poisoning, and Cushing’s disease [5].
Acute bacterial sialadenitis has three types, namely catarrhal, purulent and gangrenous. The catarrhal form is characterized by an indistinct onset, hyposalivation of the gland, swelling of the gland, spontaneous pain, or tenderness on palpation of the affected gland. There is a flow of thick saliva and a fibrin clot through the ductus. The development of the catarrhal type may lead to healing or gangrene development. The purulent type has generalized status changes, trismus, fever, stiffness, very swollen glands, acute pain, skin congestion and increased consistency. On palpation of the gland, a parotid or submandibular abscess may be palpated. The gangrenous type is a rare form, occurring in immunosuppressed patients. The skin looks red, necrotic, facial paralysis, and can cause septic shock or toxic shock syndrome [6].
Bacterial or viral infection is the most common etiology. Bacterial sialadenitis is caused by retrograde spread of infection secondary to decreased salivary flow or ductal obstruction. Decreased salivary flow may result from medications, dehydration (terminal, neonatal or postoperative illness), or general debility. Duct obstruction can be caused by sialolithiasis, strictures, or tumors. Staphylococcus aureus (gram-positive bacteria) is the most common bacterial etiology for acute submandibular sialadenitis bacterial, followed by Staphylococcus pyogenes, viridian streptococci. Gram-negative bacteria such as Hemophilus influenzae, Klebsiella species, Escherichia coli and less often anaerobic pathogens such as Peptostreptococcus spp, Prevotella, Fusobacterium, Porphyromonas, and Bacteroides also cause acute sialadenitis. The viruses that cause sialadenitis most often are paromyxovirus, coxsackie, and cytomegalovirus [1, 5, 7].
In a previous study conducted by Brook, in patients with acute suppurative sialadenitis, cultures were performed on infected areas, both in the parotid, submandibular and sublingual glands, which found predisposing factors such as dehydration, malnutrition, dental infections, immunosuppression, diabetes mellitus and drug consumption (anticholinergics, antihistamines and hypothyroidism) [8].
The study by Brook described the importance of anaerobic bacteria in acute suppurative sialadenitis, in which this organism was present in 30 (64%) of 47 patients. Anaerobic bacteria isolated or mixed with facultative organisms from all types of infected salivary glands (submandibular, sublingual and parotid). These anaerobic bacteria are the main pathogenic bacteria found in anaerobic infections in and around the oral cavity. Administration of antimicrobial therapy is an important part of the management of patients with suppurative sialadenitis [8].
The choice of antibiotic depends on the etiologic agent(s), and most cases respond to antimicrobial therapy. Broad-spectrum antimicrobial therapy is indicated for the treatment of all possible aerobic and anaerobic pathogens [8].
In this patient, the age of 9 months (infant) is one of the risk factors for the occurrence of infectious sialadenitis. Transmission of bacteria from the oral cavity to the salivary ducts and salivary glands (bacterial contamination of retrograde oral flora) and decreased saliva production due to lack of fluid intake due to coughing and colds for 10 days are also thought to be risk factors in this patient.
Clinical symptoms of acute infectious sialadenitis in this patient are acute swelling and significant tenderness of the affected gland and 30% have erythema of the gland. Systemic symptoms may also occur, which are fever, chills and malaise. On physical examination, warmth and induration were found in the affected gland [3]. In this case, the diagnosis of acute submandibular sialadenitis caused by bacterial infection can be established clinically, such as fever and swelling of the left neck, precisely under the left lower jaw. Laboratory examination in this patient (presence of leukocytosis and an increase in CRP) supports the diagnosis.
Multiple bilateral submandibular lymph node enlargement (mainly left) was shown in submandibular ultrasonography of the patient. Ultrasound examination is non-invasive, inexpensive, and useful as the first diagnostic imaging tool in establishing the diagnosis of sialadenitis. Ultrasonography is well tolerated especially for children, but the drawback of this imaging is that it is an operator-dependent technique. Ultrasound of bacterial sialadenitis detects unilateral or bilateral hypoechoic salivary gland enlargement associated with duct dilatation; whereas in advanced cases, an anechoic focus with fine and coarse echoes (abscess) may be found. Inflammatory changes around the gland and reactive cervical lymphadenopathy also occur [9]. In general, ultrasound can also detect atrophy, diffuse or localized lesions, cysts, stones, and calcifications. Ultrasonography or computed tomography (CT) scan can be performed in patients with acute sialadenitis if the patient does not improve with medication to evaluate the abscess [3]. In this case, a submandibular ultrasound was performed on the sixth day of treatment with intravenous antibiotic therapy where the patient’s condition was not feverish, and swelling was still present. FNAB examination in patients with acute bacterial sialadenitis was performed to determine the etiology (by bacteriological examination of the abscess) and to rule out differential diagnoses such as malignancy. In this patient, the results of the FNAB examination did not show a specific process or malignant tumor cells with the conclusion of non-specific chronic lymphadenitis. In this case, the sample of the bacterial culture test that was examined as a sample was blood, and it was found that there was no growth of aerobic bacteria.
The initial therapy in hospitalized patients with acute bacterial sialadenitis is empirically with broad-spectrum antibiotics that include both gram-positive and negative agents such as ampicillin sulbactam and intravenous gentamicin [3]. In this patient, the condition improved (“timeline figure”) with the administration of intravenous antibiotics ampicilin sulbactam and gentamicin for 7 days followed by oral antibiotics co-amoxiclav for 5 days while outpatient. The patient’s condition improved with empirical antibiotic therapy in line with the evaluation of a complete blood count on the sixth day of treatment where the leukocytosis improved and CRP decreased. The results of day 8 blood culture in this patient did not show the growth of aerobic bacteria.
Other treatments include rehydration, oral hygiene, stimulation of saliva with sialagogues (sour candy, lemon wedges, orange juice), and avoiding drugs that dry out mucous membranes [5]. Conservative management in this patient, in addition to hydration with maintenance infusion fluids, also gave paracetamol for analgesic and antipyretic.
Complications may include abscess, septicemia, thrombophlebitis or osteomyelitis. However, with appropriate therapy in cases of acute bacterial sialadenitis, most patients will improve and complications are rare [3]. In this patient, there were no complications during treatment or control at the polyclinic and the prognosis was good.
Conclusion
The diagnosis of acute bacterial submandibular sialadenitis can be made clinically by the presence of submandibular swelling and fever, supported by laboratory tests such as neutrophil predominance leukocytosis and elevated CRP. Ultrasonography is the initial imaging option, especially in children with cases of submandibular sialadenitis. Pus culture can establish the etiology. FNAB can help establish the etiology of the diagnosis and rule out other differential diagnoses such as malignancy. Initial therapy includes hydration, empirical broad-spectrum intravenous antibiotics for gram-positive and negative bacteria and symptoms such as analgesics and antipyretics. Other treatments include oral hygiene, stimulation of saliva with sialagogues (sour candy, lemon slices, orange juice), and avoiding drugs that make the mucous membrane dry as well as warm compresses and massage with strong external pressure starting from the angle of the mandible to the submentum. With adequate therapy, complications are rare and the prognosis is good.
Acknowledgments
The authors would like to thank the parents of the patient who have been willing to participate as research respondents for the development of science.
Financial Disclosure
Authors have no financial relationships relevant to this article to disclose.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent for publication of this report was obtained from the parents of the patient.
Author Contributions
Edi Hartoyo drafted, reviewed, revised the manuscript, and registered the case report for publication. Ira Devi Alamsyah Guntoro collected data, compiled the initial manuscript, reviewed and revised the manuscript. Yulia Margareth compiled the manuscript, reviewed and revised the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Francis CL, Larsen CG. Pediatric sialadenitis. Otolaryngol Clin North Am. 2014;47(5):763-778.
doi pubmed - Chandak R, Degwekar S, Chandak M, Rawlani S. Acute submandibular sialadenitis-a case report. Case Rep Dent. 2012;2012:615375.
doi pubmed - Djohan WH, Sapto H. Diagnosis dan penatalaksanaan sialadenitis bakteri. JIMKI. 2020;8:136-145.
doi - Firdaus IWAK, Apriasari ML. Manajemen of acute bacterial sialadenitis. Dentino. 2018;3:7-9.
- Schlieve T, Kolokythas A, Miloro M. Salivary Gland Infections. In: James RH, Elie MF (eds). Head, Neck, and Orofacial Infections. Elsevier: St. Louis. 2016:232-247.
doi - Toma A, Furfui P, Chirila A, Oancea ALA, Grigore R, Bertesteanu SVG. Clinical diagnosis and treatment of salivary glands inflammation. Archives of the Balkan Medical Union. 2019;54:712-719.
doi - Krishnamurthy S, Vasudeva SB, Vijayasarathy S. Salivary gland disorders : A comprehensive review. World J Stomatol. 2015;4:56-71.
doi - Brook I. Aerobic and anaerobic microbiology of suppurative sialadenitis. J Med Microbiol. 2002;51(6):526-529.
doi pubmed - Abdel Razek AAK, Mukherji S. Imaging of sialadenitis. Neuroradiol J. 2017;30(3):205-215.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
