| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Case Report
Volume 11, Number 2, June 2022, pages 56-59
Moraxella osloensis Bacteremia and Pneumonia in a Seventeen-Year-Old Patient
Holden W. Richardsa, e, Christina Lancionia, b, Michael Jonesc, d
aOregon Health and Science University School of Medicine, Portland, OR, USA
bDivision of Infection Diseases, Department of Pediatrics, Oregon Health and Science University, Portland, OR, USA
cDepartment of Pediatrics, Oregon Health and Science University, Portland, OR, USA
dDepartment of Pediatrics, PeaceHealth Sacred Heart Riverbend Hospital, Springfield, OR, USA
eCorresponding Author: Holden W. Richards, Oregon Health and Science University School of Medicine, Portland, OR 97239, USA
Manuscript submitted February 27, 2022, accepted May 3, 2022, published online June 2, 2022
Short title: Moraxella osloensis Bacteremia and Pneumonia
doi: https://doi.org/10.14740/ijcp483
| Abstract | ▴Top |
Moraxella osloensis (M. osloensis) is a rare cause of bacteremia and pneumonia in pediatric patients. The published case reports of M. osloensis bacteremia are limited. Among children, there are only seven cases of invasive disease from M. osloensis identified, and specific treatment guidelines are not available. Our case adds to the limited literature on this rare bacterium and highlights the need to better define antimicrobial resistance patterns. Here we report a case of a 17-year-old previously healthy female admitted with M. osloensis bacteremia and pneumonia. The patient initially presented with clinical and radiographic findings consistent with community-acquired pneumonia and was sent home after an emergency department (ED) visit with oral antibiotic treatment. She was advised to return for admission after the blood culture obtained in the ED reported growth and was noted to have persistent symptoms despite antibiotic adherence. Treatment with ceftriaxone for 3 days intravenously followed by 7 days of oral cefdinir resulted in rapid clinical improvement and resolution of her infection. Due to its sparse prevalence in the literature, there is currently no standard antibiotic regimen for treatment of invasive infections with M. osloensis; a variety of therapies ranging from 7 to 14 days in duration have been successfully employed. While Moraxella species (e.g., Moraxella catarrhalis (M. catarrhalis)) are typically β-lactamase producing, reports regarding β-lactamase production by M. osloensis have been inconsistent. M. osloensis is a rare cause of invasive infection in adolescents. Given limited published data regarding antibiotic sensitivities of this specific organism, microbiologic sensitivities should be obtained if possible and response to therapy should be closely monitored.
Keywords: Moraxella; Moraxella osloensis; Bacteremia; Pneumonia; Infectious disease
| Introduction | ▴Top |
The Moraxella genus of gram-negative bacteria originates from the Moraxellaceae family, which is notable for common human and bovine pathogens (Moraxella catarrhalis (M. catarrhalis) and Moraxella bovis, respectively) [1]. M. catarrhalis typically produces a β-lactamase, and treatment of these typical pathogens has been well established. In children and adolescents, the usual manifestations of M. catarrhalis, specifically otitis media and sinus infections, commonly respond to treatment with amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and cephalosporins, depending on regional susceptibility [2]. Conversely, Moraxella osloensis (M. osloensis), an aerobic gram-negative coccobacillus, does not have a standardized treatment due to its rarity.
M. osloensis has been isolated from environmental sources, and there have been few reports of the organism causing human disease in older and/or immunocompromised patients [3, 4]. Among previously healthy children and adolescents, M. osloensis infection has been rarely reported. In total, there are fewer than 20 pediatric cases and around 30 adult infections with this species reported, with adults experiencing more severe disease [4]. In children, only seven cases of bacteremia have been reported with this species [4]. This case report is presented to build upon the scarce literature of invasive M. osloensis bacteremia in children and to outline antibiotic recommendations for treatment for this species.
| Case Report | ▴Top |
Investigations
A 17-year-old previously healthy adolescent female presented to the emergency department (ED) with complaints of chills, body aches, mild headache, and bilateral eyelid swelling noted upon wakening that morning. There was no associated neck stiffness, neck pain, shortness of breath, abdominal pain, rash, or dysuria. She had been well with no symptoms prior to initial presentation. There were no sick contacts and no recent travel. She was working on a flower farm over the summer and her last day was 7 days prior to admission. She lived with her parents and brother. She would be attending 12th grade at a local public high school. She denied taking any medications and had no prior hospitalizations or underlying medical conditions. Family history was significant for maternal grandparents with arthritis, but there was no history of autoimmune conditions like systemic lupus erythematosus, rheumatoid arthritis, or individuals with immunodeficiencies. The patient was up-to-date on her vaccinations and received her first dose of the AztraZeneca coronavirus disease 2019 (COVID-19) vaccine 1 week prior to presentation.
On presentation, the patient was febrile and tachycardic, with a temperature of 38.1 °C and pulse of 169 beats per minute. Blood pressure was 135/82 mm Hg, respiratory rate of 24 breaths per minute, and oxygen saturation of 99% on room air; body mass index (BMI) was 33.45 kg/m2. Physical exam was notable for edema of bilateral eyelids with faint clear discharge and minimal conjunctival injection from both eyes. Cardiac exam demonstrated tachycardia without associated murmur, friction rub, or gallop rhythm. The remained of examination was unremarkable.
Diagnosis
Initial laboratory tests revealed: neutrophilia (9.1 × 103/µL) and lymphopenia (0.4 × 103/µL) on complete blood count (CBC), minor uncompensated respiratory alkalosis (pH 7.45, pCO2 33 mm Hg, HCO3 22 mmol/L) on venous blood gas, negative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction (PCR) from nasal swab, negative urine pregnancy test, normal troponin, normal thyroid-stimulating hormone, as well as unremarkable comprehensive metabolic panel and urinalysis. Two blood cultures were collected. Chest X-ray (Fig. 1) demonstrated a nonspecific small focus of ground-glass opacity in the periphery of the left lung. electrocardiogram (ECG) demonstrated sinus tachycardia with left atrial enlargement; subsequent transthoracic echocardiogram was normal.
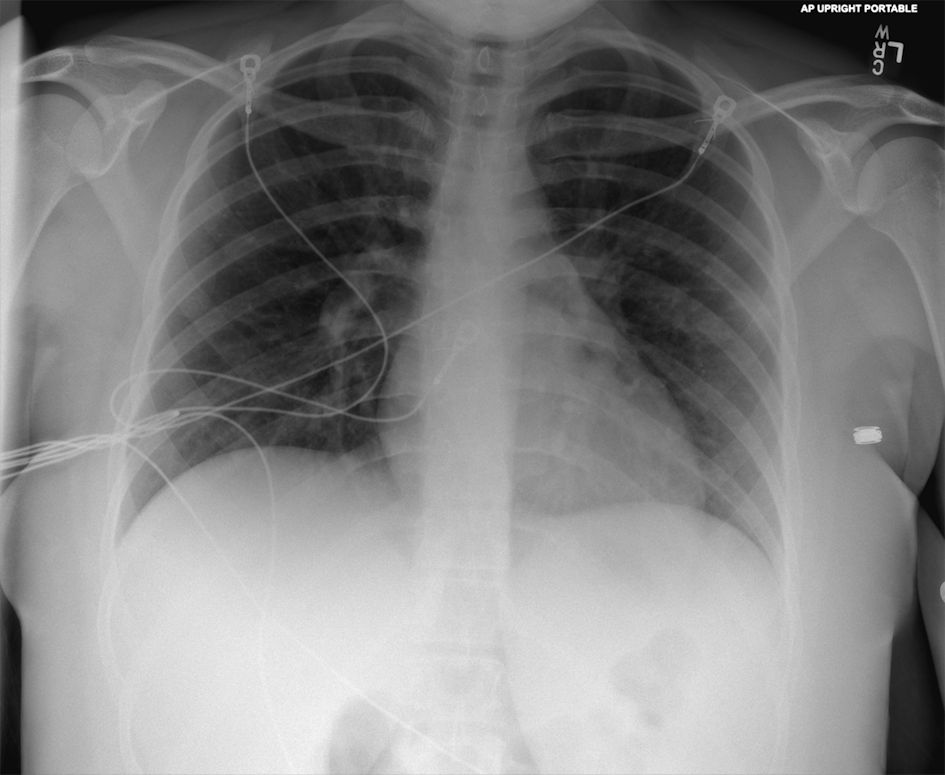 Click for large image | Figure 1. Anteroposterior (AP) chest X-ray from initial emergency department visit. |
Patient was diagnosed with viral syndrome, conjunctivitis, and pneumonia of the left lung. Due to concerns for secondary bacterial infection, a prescription for amoxicillin-clavulanate (875/125 mg) tablets and ofloxacin 0.3% ophthalmic drops was provided, and the patient was discharged home. Two days later, patient was instructed to return to the ED as one of two blood cultures obtained in ED was reported positive. Upon arrival, patient was noted to have persistence of intermittent fevers, headache, bilateral eye swelling and redness, as well as cough and congestion, despite compliance with oral antibiotics.
Repeat physical exam on admission demonstrated persistence of tachycardia with pulse of 95 beats per minute. She was alert, conversant, and nontoxic. She had mildly erythematous and edematous upper eyelids bilaterally, crusting in the eyelashes, and clear watery discharge from both eyes with minimal conjunctival injection. Cardiovascular, she was tachycardic, no murmur, and capillary refill time of 2 s.
There were diminished breath sounds at the bases of her lungs with no focal crackles, but no retractions, or increased work of breathing. Extremities were slightly cool to touch, but there was no edema and there was 2+ distal pulses. The remainder of her physical exam was normal.
Repeat laboratory evaluations on admission documented resolved neutrophilia with persistent lymphopenia (0.5 × 103/µL), and an elevated C-reactive protein (8.9 mg/dL; normative value < 1.0 mg/dL). Lactate was normal and multiplex, molecular panel for common respiratory viruses was negative. Repeat chest X-ray (Figs. 2, 3) demonstrated slightly increased bilateral perihilar linear opacities compared to prior exam.
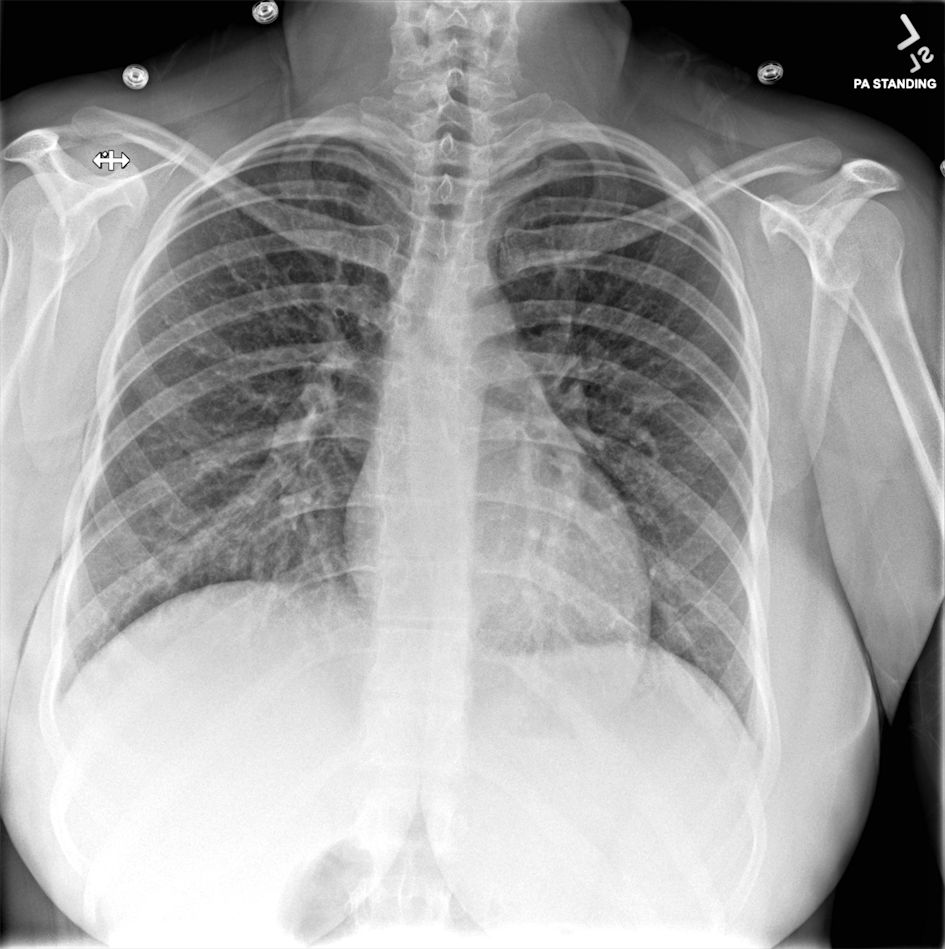 Click for large image | Figure 2. Posteroanterior (PA) standard chest X-ray from second emergency department visit 2 days later. |
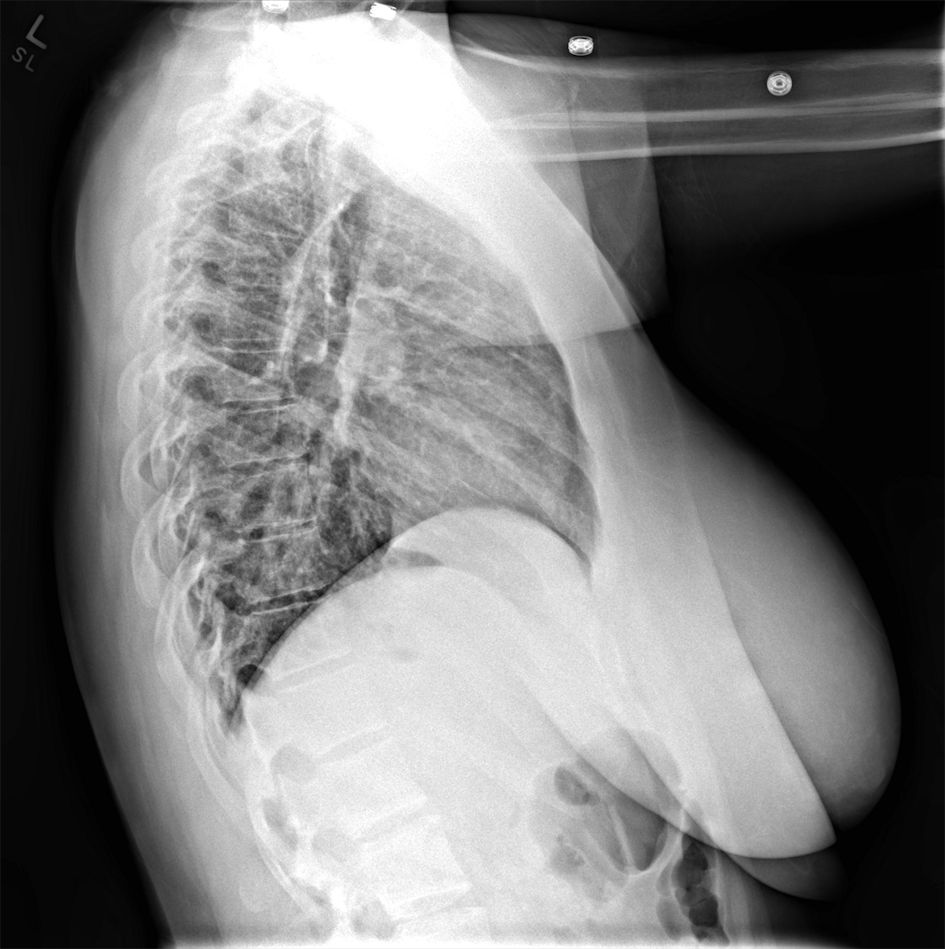 Click for large image | Figure 3. Lateral chest X-ray from second emergency department visit 2 days later. |
Initial blood cultures were reported positive for gram-negative bacilli in the aerobic bottle, with molecular diagnostics negative for common gram negatives including Escherichia coli, Klebsiella, Proteus, Serratia, and Pseudomonas. Patient was admitted for gram-negative bacteremia and pneumonia and started on empiric treatment with ceftriaxone. The following day, the organism from the blood culture was identified as M. osloensis. Antibiotic susceptibility testing was performed by Quest Diagnostics Infectious Disease using a validated assay pursuant to the Clinical Laboratory Improvement Amendment (CLIA) regulations.
Treatment
The patient was initially managed with intravenous (IV) ceftriaxone (2 g IV, every 24 h) along with maintenance fluids. A pediatric infectious disease consult was obtained when M. osloensis was identified as the causative organism to guide therapy and address concerns related to her lymphopenia upon presentation. An immunodeficiency workup was considered but ultimately deferred given the patient’s overall well appearance, immediate response to broad-spectrum antibiotics, and lack of antecedent chronic infections. The patient remained afebrile during her hospital stay and continued ceftriaxone for 2 days until discharge. Repeat blood cultures obtained on second ED visit (prior to initiation of ceftriaxone) were negative. At the time of hospital discharge, the patient was well-appearing with no increased work of breathing despite mild rhonchi still present in the lower lobes of her lungs. Her lymphocytes had mildly improved to 0.8 × 103/µL. Susceptibilities of the M. osloensis were reported as sensitive to all antibiotics tested including ceftriaxone, cefepime, ceftazidime, ciprofloxacin, gentamicin, tobramycin, and trimethoprim-sulfamethoxazole. The laboratory did not indicate if the organism produced β-lactamase, and sensitivity to ampicillin was not reported.
On the day of hospital discharge, the patient was transitioned to 7 days of cefdinir (300 mg twice a day (BID)) to complete a total 10-day treatment course for M. osloensis bacteremia and pneumonia.
Follow-up and outcomes
A follow-up visit with her primary care physician (PCP) was recommended to obtain a repeat CBC with differential 1 - 2 weeks post-discharge to establish resolution of lymphopenia. It was also recommended that an infectious disease outpatient referral be completed if lymphopenia persisted. The patient was evaluated by her PCP 2 weeks after discharge and was completely asymptomatic at that time. A repeat CBC obtained at this visit demonstrated resolution of lymphopenia.
| Discussion | ▴Top |
Here we present a rare case of invasive M. osloensis infection resulting in bacteremia and pneumonia in an otherwise healthy adolescent who failed to respond to initial outpatient antibiotic therapy. M. osloensis has generally been considered a commensal organism and rare cause of invasive disease. As observed in this case, it is generally not recognized by most commercial bacterial identification systems, and due to its sparse prevalence in the literature, there is currently no standard antibiotic regimen for treatment of invasive infections [4]. Moreover, while the more common Moraxella species (e.g., M. catarrhalis) are typically β-lactamase producing, this has been inconsistently reported among clinical M. osloensis isolates [5, 6]. This uncertainty is illuminated by case reports showing sensitivities and effective treatment of this species in children with penicillin and ampicillin, which should not be effective in a β-lactamase producing organism [3, 4]. Some reported cases, however, have required multiple trials of different antibiotics to achieve effective therapy. Here, the patient failed initial outpatient antibiotics with amoxicillin-clavulanate, a surprising finding given the effectiveness of this antibiotic against other β-lactamase producing Moraxella species. In pediatric case reports, children were most commonly empirically started on cephalosporin-based therapy and in these cases no secondary line therapy has been required. Ultimately, clinicians should request susceptibilities be performed on M. osloensis isolates to ensure that an effective antibiotic is provided, and to avoid use of a broad-spectrum antibiotic if not necessary.
Learning points
M. osloensis is a rare cause of bacteremia and pneumonia in both the adult and pediatric literature. M. osloensis may have unique susceptibilities to antibiotics as compared to other Moraxella species. Antimicrobial susceptibility testing should be requested, and response to empiric therapy should be closely monitored to ensure disease resolution [5, 6].
Acknowledgments
We would like to thank our patient who approved the writing of this case report and for the utilization of the images.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
Holden W. Richards was responsible for writing the first draft of the paper, incorporating revisions, editing figures and manuscript preparation. Dr. Christina Lancioni revised the manuscript for important intellectual content and approved the final version. Dr. Michael Jones was responsible for revising the manuscript for intellectual content and approved the final version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Garrity GM. The proteobacteria, Part B: The Gammaproteobacteria. In: Auflage, ed. Bergey's manual of systematic bacteriology. Vol 2. New York: Springer; 2005.
- TF M. Moraxella catarrhalis, Kingella, and other gram-negative cocci. In: Mandell GL BJ, Dolin R, ed. Mandell, Douglas, and Bennett's principles and practices of infectious diseases. Vol 2. 7 ed. Philadelphia: Churchill Livingstone Elsevier; 2010; p. 2771.
doi - Maruyama Y, Shigemura T, Aoyama K, Nagano N, Nakazawa Y. Bacteremia due to Moraxella osloensis: a case report and literature review. Braz J Infect Dis. 2018;22(1):60-62.
doi pubmed - Tabbuso T, Defourny L, Lali SE, Pasdermadjian S, Gilliaux O. Moraxella osloensis infection among adults and children: A pediatric case and literature review. Arch Pediatr. 2021;28(4):348-351.
doi pubmed - Han XY, Tarrand JJ. Moraxella osloensis blood and catheter infections during anticancer chemotherapy: clinical and microbiologic studies of 10 cases. Am J Clin Pathol. 2004;121(4):581-587.
doi - Alkhatib NJ, Younis MH, Alobaidi AS, Shaath NM. An unusual osteomyelitis caused by Moraxella osloensis: A case report. Int J Surg Case Rep. 2017;41:146-149.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
