| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Case Report
Volume 11, Number 2, June 2022, pages 39-44
Possible Relation of Skin and Nail Changes in an Infant to COVID-19 Infection
Riya K. Kalraa, c , Nicole L. Lewisb, Stacey A. Shubecka, Kerry P. Mychaliskaa
aDepartment of Pediatrics, Beaumont Hospital, Royal Oak, MI, USA
bOakland University William Beaumont School of Medicine, Rochester, MI, USA
cCorresponding Author: Riya K. Kalra, Department of Pediatrics, Beaumont Children’s Hospital, Royal Oak, MI 48073, USA
Manuscript submitted April 1, 2022, accepted April 21, 2022, published online June 27, 2022
Short title: Skin and Nail Changes in a COVID-19 Infant
doi: https://doi.org/10.14740/ijcp481
| Abstract | ▴Top |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first reported in China in December 2019, rapidly developed into a global pandemic and public health emergency. A variety of cutaneous manifestations associated with coronavirus disease 2019 (COVID-19) have been described in the literature, including urticarial, maculopapular, papulovesicular, purpuric eruptions, livedo reticularis, and thrombotic ischemic lesions. COVID toes, or acute COVID-19-associated chilblains, have been reported as a manifestation of COVID-19 in teenagers and children, as young as 5 years old. We are presenting a case of an 11-month-old female who presented to our clinic with two distinct skin changes following COVID-19 infection: one, COVID-19-related toes, fingers, and nails, and two, exacerbation of atopic dermatitis secondary to COVID-19. To our knowledge, this is the first case of skin and nail manifestation in an infant following COVID-19 infection. This case also supports the current literature that atopic dermatitis can be triggered by COVID-19 infection in predisposed individuals.
Keywords: COVID-19; Chilblains; Atopic dermatitis
| Introduction | ▴Top |
Novel coronavirus (severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)), first reported in China in December 2019, rapidly developed into a global pandemic and public health emergency. Although minimal dermatologic findings were reported at the initial outbreak, more recently, a variety of cutaneous manifestations associated with coronavirus disease 2019 (COVID-19) have been described in the literature, including urticarial, maculopapular, papulovesicular, purpuric eruptions, livedo reticularis, and thrombotic ischemic lesions [1]. Acute chilblains, one of the most common skin manifestations, were initially observed in adults; however, teenagers and children, as young as 5 years age [1, 2], did not remain immune to this. It is a mildly symptomatic condition with an excellent prognosis, usually requiring no therapy, but the etiopathogenesis remains unknown [2]. We are presenting a case of an 11-month-old female who presented to our clinic with signs and symptoms consistent with COVID-19-related toes, fingers, and nails, and exacerbation of atopic dermatitis 3 months after the initial presentation.
| Case Report | ▴Top |
Investigations
An 11-month-old fully vaccinated female, with past medical history of atopic dermatitis, was brought in by her mother for painless, brittle toenails and fingernails. Mom first noticed these nails 4 - 5 days prior to presentation when she went to cut the patient’s nails and one “cracked”. There was no fever, rashes, cough, difficulty breathing, rhinorrhea/congestion, ear pulling/discharge, vomiting, diarrhea/constipation, or known sick contacts at the time of presentation. However, there was a history of recent travel to Connecticut (> 10 days prior to presentation) but no hiking, walking in the woods, or bug/tick bites were reported. She lives at home with her parents and siblings, none of whom had similar symptoms. There was no other relevant social and environmental history. There was no history of recent exposure to cold temperatures. There was no change in her activity/appetite/sleep or bowel/bladder habits since the onset of symptoms. Patient’s medications include only a daily vitamin D supplement, and occasional Zyrtec and hydrocortisone ointment for atopic dermatitis flare. The only notable history in the patient was COVID-19 infection 6 months prior to presentation (at 5 months of age), when she presented with fevers for 1 day (maximum temperature 38.3 °C) and vomiting for 5 days. She tested positive for COVID-19 by SARS-CoV-2 (COVID-19) nucleic acid amplification (NAA) (reverse transcription-polymerase chain reaction (RT-PCR)). She was quarantined for 2 weeks, during which her symptoms started improving, and completely resolved in the weeks that followed without intervention or complications. No one in the family was tested as they remained asymptomatic. There were no residual signs/symptoms related to COVID-19 at the time of presentation. There is no family history of autoimmune diseases or genetic conditions affecting skin, nails, or hair, although mom did recall a personal history of similar features in one fingernail when she was under 3 years of age which resolved on its own. She was born at gestational age of 39 weeks and 1 day by a repeat cesarean section in a pregnancy complicated by gestational thrombocytopenia. The perinatal course was complicated by meconium-stained amniotic fluid, but was otherwise uncomplicated without need for resuscitation, with appearance, pulse, grimace, activity, respiration (APGAR) score of 9 at 1 min and 5 min. Birth weight was 3.32 kg (57th percentile for age), length was 49.5 cm (58th percentile for age) and head circumference was 33.5 cm (37th percentile for age). On physical examination, she was at the 50th percentile for height and weight, and at the 70th percentile for head circumference. Brittle toenails were noted, both of the first toes being the most affected. The skin around bilateral first toes was dry and erythematous. Fingernails were not as affected, but the skin around the fingernails appeared xerotic. Mom stated that previously the skin around the patient’s nails was initially blue and later turned red. At the time of presentation, the bluish discoloration had resolved, and the nails appeared brittle and dry as demonstrated in Figures 1, 2. The rest of the physical examination, including the neurological examination, was completely normal for her age.
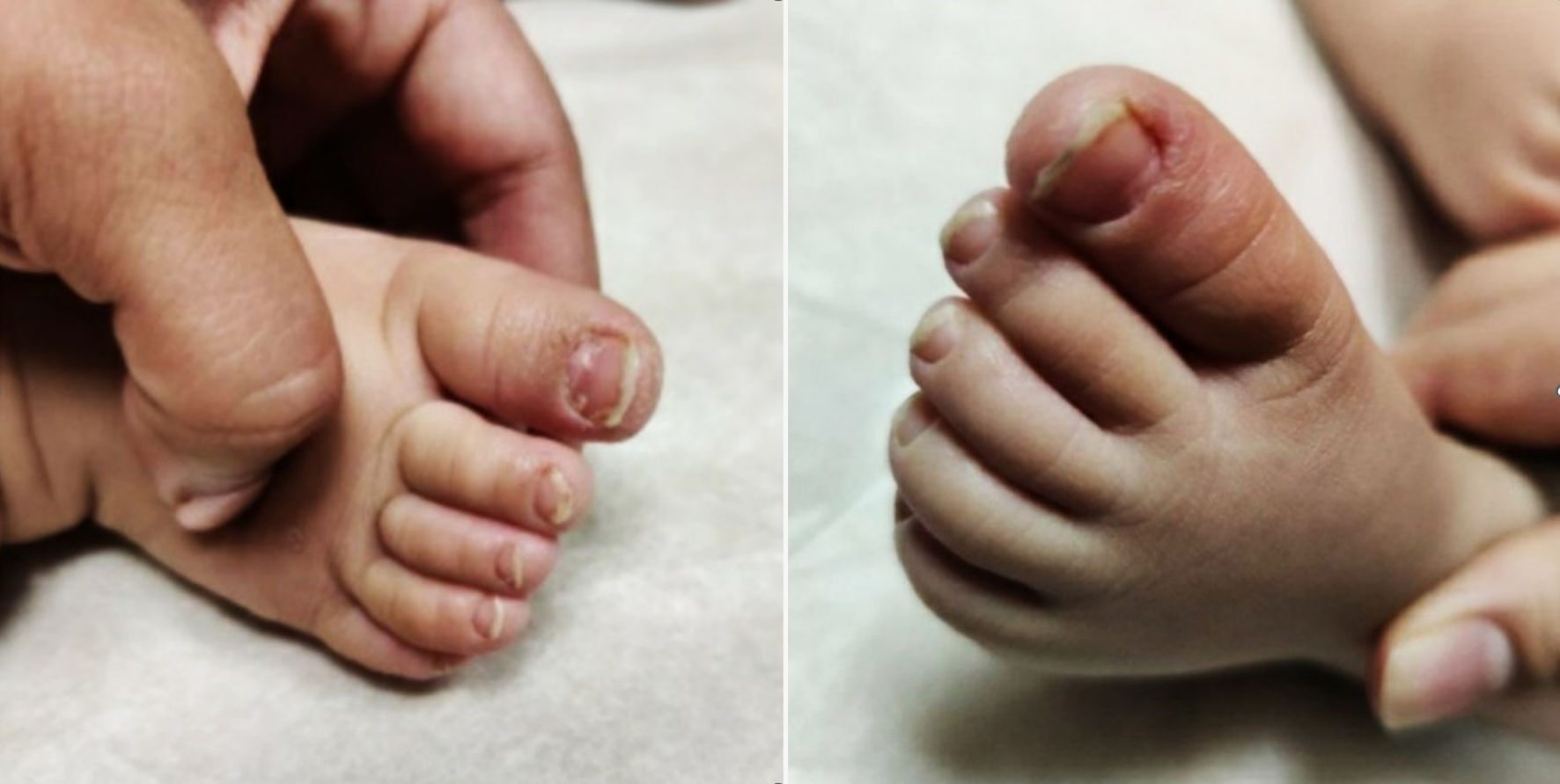 Click for large image | Figure 1. Feet with xerosis around the nails (first visit). |
 Click for large image | Figure 2. Hands with xerosis around nails (first visit). |
Diagnosis
Our differential diagnosis included (but was not limited to) COVID-19-related skin and nail manifestation, onychomadesis, onychomycosis, paronychia, nutrient deficiency, medication side effect, autoimmune disease, trauma, and ectodermal dysplasia. Other diagnoses listed in Figure 3 were also considered [1]. All these were discussed with the parent and salient features of each were discussed. Based on the temporal relationship between her symptoms and timing of COVID-19 infection, along with the history (especially the bluish discoloration that the patient’s mom described) and physical exam findings, we concluded that her nail and skin changes were most likely a sequela of her previous COVID-19 infection.
 Click for large image | Figure 3. Differential diagnosis of COVID-19-related chilblains. COVID-19: coronavirus disease 2019. |
Treatment
As her symptoms were most likely secondary to COVID-19 infection, no treatment was deemed necessary except for regular moisturization. No laboratory investigations or imaging studies were done as they were not necessary at that time. Mom was given recommendations to return to clinic if patient’s nails or skin around the nails become erythematous, cyanotic, warm or if there is any additional change in appearance.
Follow-up and outcomes
At follow-up 10 days later, mother reported that patient had been doing well with no concerns since the previous visit. Per mom, the appearance of patient’s nails and skin around the nails had improved which is demonstrated in the Figures 4, 5. She continued to feed, void, and stool appropriately. Additionally, there were no changes in activity or sleep, and she remained afebrile.
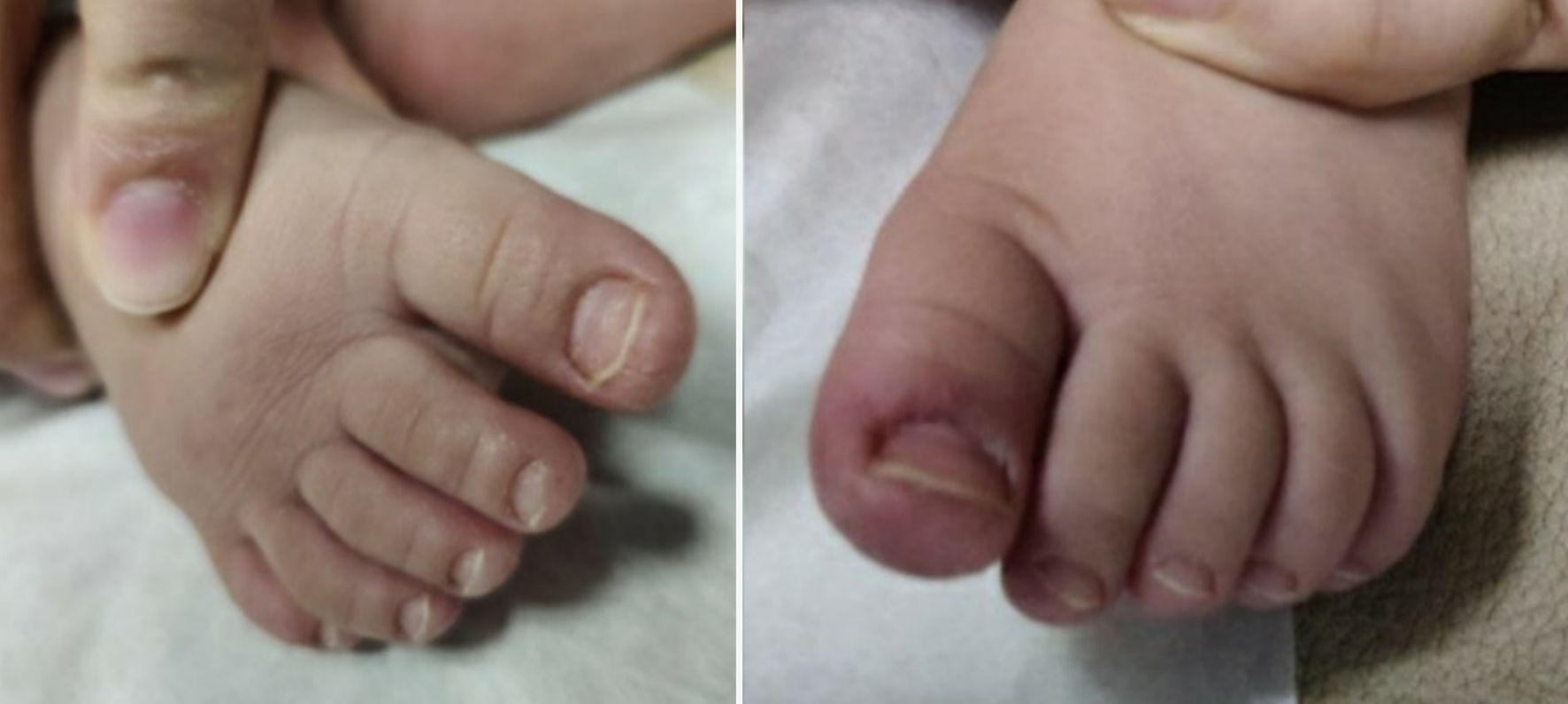 Click for large image | Figure 4. Feet on follow-up visit. |
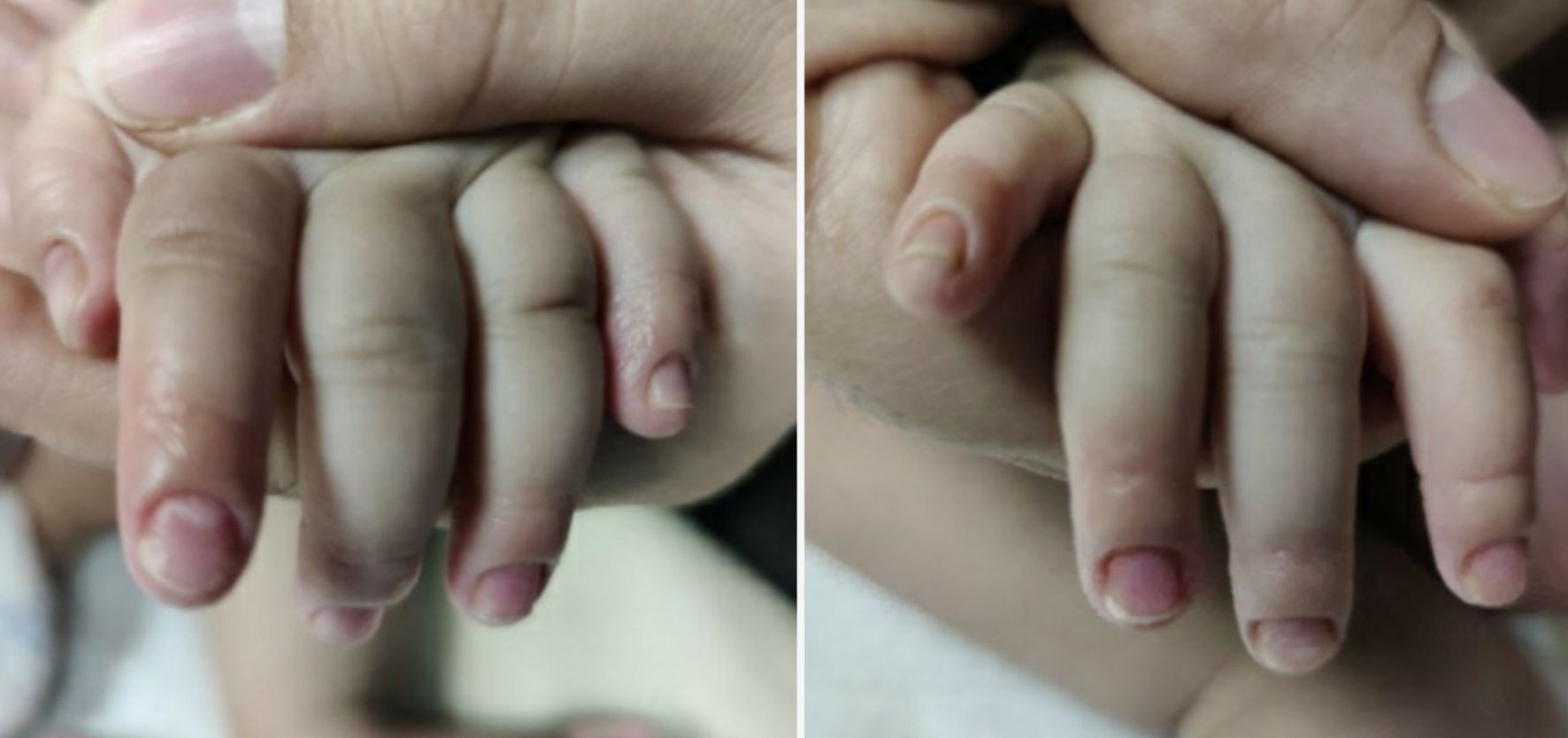 Click for large image | Figure 5. Hands on follow-up visit. |
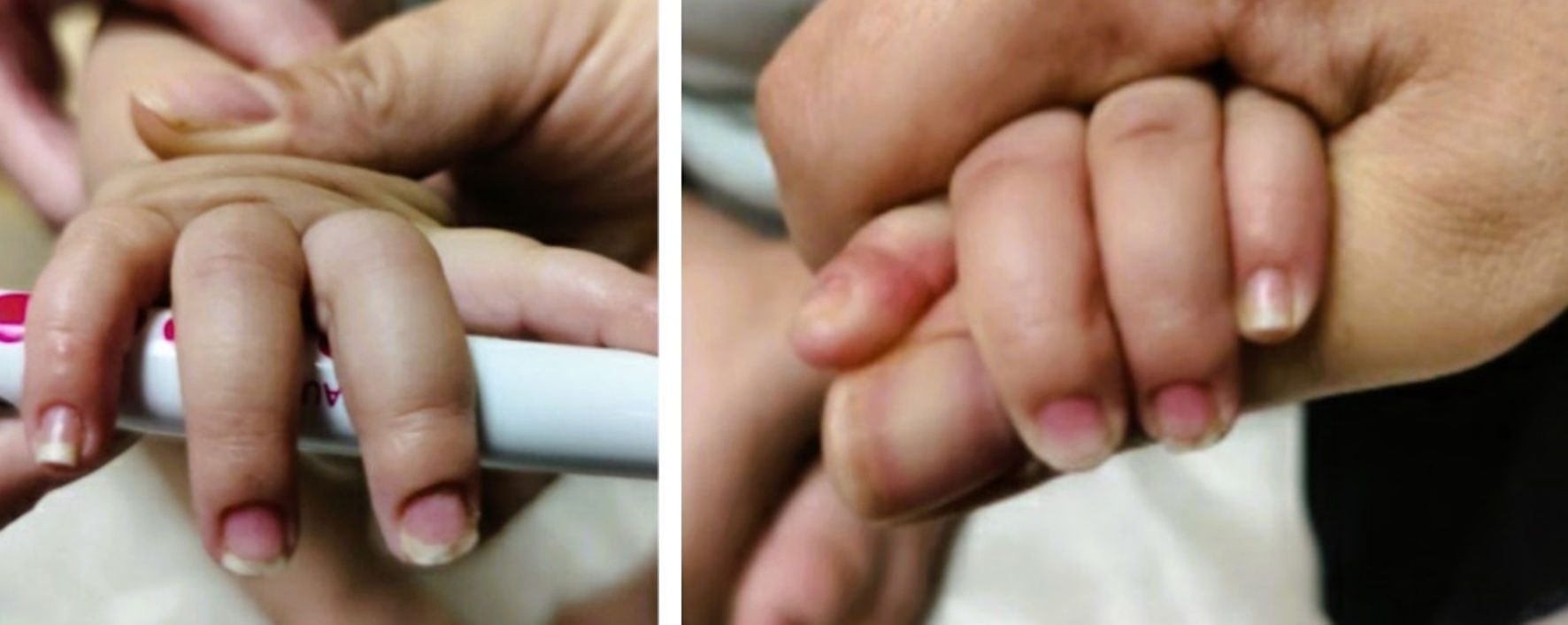
Subsequently, 3 months later, the patient presented a distinctly different rash that consisted of erythematous dry plaques consistent with dyshidrotic eczema of all fingers and thumbs (Fig. 6).
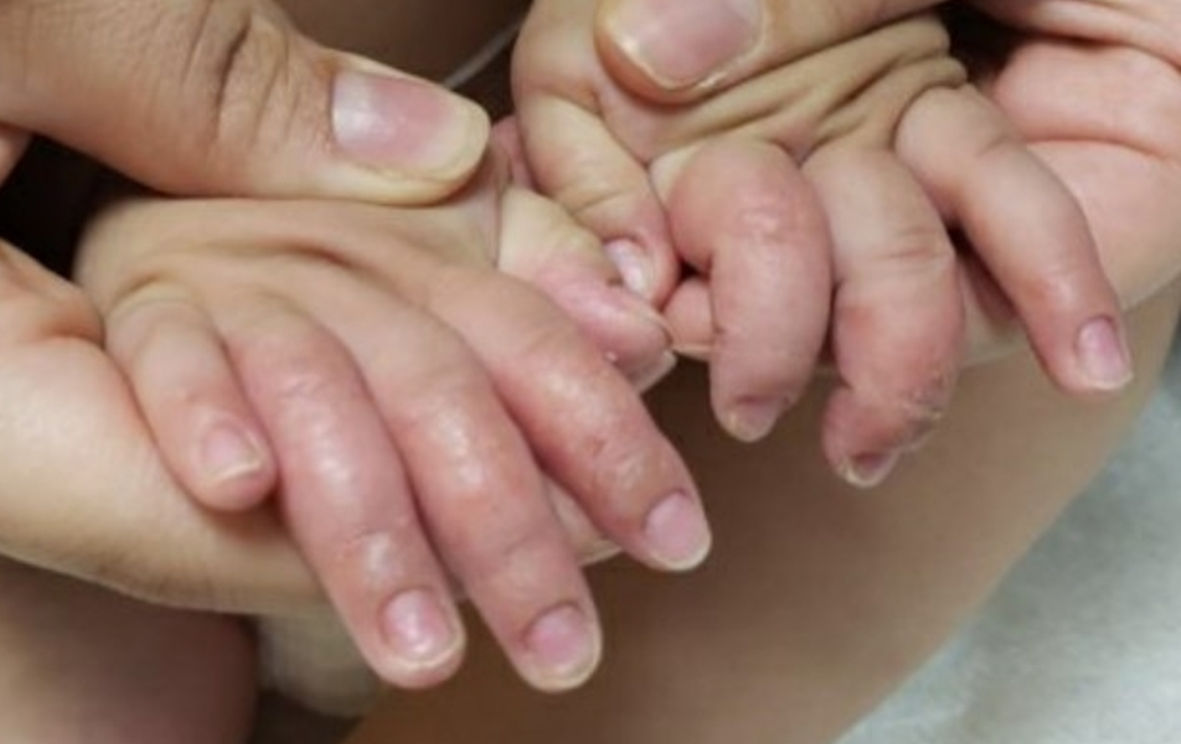 Click for large image | Figure 6. Dyshidrotic eczema over fingers and thumbs on final follow-up. |
| Discussion | ▴Top |
An 11-month-old fully vaccinated female with atopic dermatitis presented with painless, brittle toenails and fingernails. After careful review of her history, physical exam and the differential diagnoses, her skin changes seemed to be secondary to her past COVID-19 infection. On further follow-up, she was seen to have dyshidrotic eczema of all fingers and thumbs. This finding is consistent with the published data documenting COVID-19-associated surge of atopic dermatitis [3, 4], which is not yet documented in this age group.
Novel coronavirus, first reported in China in December 2019, rapidly developed into a global pandemic and public health emergency. Although the most common symptoms of cough, fever, chills, respiratory distress, and myalgias reported with this infection are well documented, there are numerous dermatologic manifestations that may be less recognized, particularly in infants. In the literature, several cutaneous manifestations have been associated with COVID-19, such as maculopapular, papulovesicular, urticarial, thrombotic ischemic lesions, purpuric eruptions, and livedo reticularis [5]. One such dermatologic manifestation, colloquially known as “COVID toes” is described above in an infant. Most patients who have presented with these are adolescents and children, as young as 5 years. These findings resemble perniosis or chilblains, a rash that is typically associated with exposure to cold conditions [6, 7]. While primary perniosis is idiopathic, secondary perniosis is triggered by an underlying systemic cause, resulting in vasospasm and ischemia which leads to painful, pruritic erythrocyanotic discoloration and swelling. Various autoimmune disorders have been implicated, such as systemic lupus erythematosus, sarcoidosis, vasculitides, antiphospholipid disease, among others [7]. It is possible that this mechanism is responsible for COVID toes; however, personal and family history of autoimmune disease and recent exposure to cold temperatures should still be considered in patients presenting with chilblain-like lesions.
Kolivras et al [8] further hypothesized the etiopathogenesis of this condition in their case report. They proposed that young patients exhibit an early interferon I (IFN-I) response as compared to the older patients who have an inadequate or delayed response. The former mutes early viral replication but induces microangiopathic changes which ultimately produce a chilblain-like eruption, while an inadequate or delayed response exacerbates preexisting hypercytokinemia, leading to increased morbidity and mortality. From this, Kolivras et al [8] concluded that early IFN-I response may be advantageous in younger population, while the delayed or insufficient response may be disadvantageous in older patients. This theory can explain why COVID-19 infection-induced chilblains have a self-limiting course with good prognosis in children and adolescents.
Chilblain lesions identified during COVID-19 infection tend to be self-limiting. A retrospective review and follow-up of 22 children and adolescents by Andina et al [9] who presented with COVID toes in Spain in April 2020 revealed that the symptoms generally started to fade 7 - 10 days after presentation and were markedly improved or completely resolved after 3 - 5 weeks in all patients spontaneously. Another retrospective review of 26 children [10] with COVID toes in Italy in March - April 2020 also demonstrated that all patients recovered without any treatment. Although patients have been reported to have received various symptomatic treatments, such as topical heparin, topical nitroglycerin, topical and systemic corticosteroids, to date, no randomized control trials have been performed to assess the efficacy of these interventions [6, 7, 9, 10].
All the diagnoses discussed in the “diagnosis” section above were carefully considered and excluded. Onychomadesis, proximal separation of the nail plate from the matrix due to temporary cessation of nail growth post-viral infection, has been seen in a 47-year-old woman 3 months after COVID infection [2]. In our patient, physical exam did not suggest this diagnosis. The physical exam was also not consistent with onychomycosis, as there was no thickening or discoloration of nails. The patient had been feeding well without concerns and was taking daily iron-containing formula, so nutrient deficiency was excluded. She was also not taking any medication commonly associated with nail changes (anticonvulsants, chemotherapeutic agents, lithium, or retinoids [11]). Her young age, negative family history of autoimmunity, and no signs/symptoms suggestive of an autoimmune disease in the patient rendered the diagnosis of an underlying autoimmune disorder very unlikely. Trauma was excluded as there was no history of injury and no mechanism that would explain the involvement of all her nails. Paronychia and other infectious etiologies were excluded as there was no inflammation of nail folds and the symptoms were resolving without treatment. Lastly, ectodermal dysplasia was excluded as she had no signs or symptoms consistent with this diagnosis, i.e., no skin changes, normal dentition, and thick lustrous hair.
Our patient tested positive for COVID-19 6 months prior to presentation. Although the timeline between the infection and her presentation may appear long, at this juncture, there is not enough data available regarding COVID-19 sequelae in infants and children. Furthermore, this patient subsequently presented with dyshidrotic eczema which has now been established in adult literature as being related to COVID-19 infection [3, 4]. Moreover, there is no laboratory test that can provide confirmation. This patient’s return with dyshidrotic eczema was further consistent with the ongoing inflammatory response of COVID-19 in skin and nails. This case presentation alerts the pediatric provider to document the dermatologic findings of COVID-19 and their subsequent sequelae, which will be helpful in understanding the evolution of dermatologic changes of COVID-19 in pediatric patients.
Conclusions
Acute COVID-19-associated chilblains (“COVID toes”) have been reported as a manifestation of COVID-19 in teenagers and children, as young as 5 years. This case describes COVID-19-related skin and nail manifestations in an infant, which to the best of our knowledge, has not yet been reported in the literature. Most pediatric patients who present with COVID-19 skin and nail findings are negative for COVID-19 infection on polymerase chain reaction (PCR) testing, and all have reported an asymptomatic or minimally symptomatic COVID-19 disease course. These lesions tend to be self-limiting. Although various symptomatic treatments have been discussed, no trials have been performed to assess their efficacy. With the current surge in COVID-19 infections in the pediatric population, clinicians should be aware of signs and symptoms of acute illness as well as sequelae of COVID-19 and continue to update the medical literature for better understanding of this disease in children. COVID-19 has also been reported to be associated with a surge of atopic dermatitis in predisposed individuals due to its ongoing inflammatory response. It is important for pediatric literature to report such findings seen in the adult population and continue to monitor patients for any other sequelae of continued inflammation.
Acknowledgments
We are deeply grateful to Sehaj Singh Kalra (Computer Science undergraduate student at Queen’s University, Canada) for his help with the intricacies of editing this manuscript and for his patience with the constant changes. Our heartfelt thank you to Dr. Daniel Domijan (Internal Medicine/Pediatrics resident at Beaumont Hospital, Royal Oak, MI) for his cooperation in collecting patient information at follow-up visits and bearing with our innumerable questions. We would also like to appreciate the help of our wonderful medical students at Oakland University William Beaumont School of Medicine, MI for their help with this manuscript.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose. This report does not contain a discussion of an unapproved/investigative use of a commercial product/device.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
A written informed consent was obtained from the mother of the patient after discussion of the consent paperwork in her preferred language (Arabic) with the help of a translator.
Author Contributions
Dr. Riya K. Kalra saw the patient in the clinic, obtained the consent and photographs, drafted the initial manuscript, and reviewed and revised the final manuscript. Dr. Stacey A. Shubeck and Nicole L. Lewis saw the patient in the clinic, reviewed and revised the manuscript. Dr. Kerry P. Mychaliska critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Data Availability
The authors declare that data supporting the findings of this study are available within the article and the references provided.
Abbreviations
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; COVID-19: coronavirus disease 2019; IFN-I: interferon I; NAA (RT-PCR): nucleic acid amplification (reverse transcription-polymerase chain reaction); PCR: polymerase chain reaction:
| References | ▴Top |
- Ladha MA, Luca N, Constantinescu C, Naert K, Ramien ML. Approach to chilblains during the covid-19 pandemic [Formula: see text]. J Cutan Med Surg. 2020;24(5):504-517.
doi pubmed - Senturk N, Ozdemir H. Onychomadesis following COVID-19 infection: is there a relationship? Dermatol Ther. 2020;33(6):e14309.
doi - COVID-19-associated surge of atopic dermatitis. EBioMedicine. 2021;64:103268.
doi pubmed - Buhl T, Beissert S, Gaffal E, Goebeler M, Hertl M, Mauch C, Reich K, et al. COVID-19 and implications for dermatological and allergological diseases. J Dtsch Dermatol Ges. 2020;18(8):815-824.
doi - Koschitzky M, Oyola RR, Lee-Wong M, Abittan B, Silverberg N. Pediatric COVID toes and fingers. Clin Dermatol. 2021;39(1):84-91.
doi pubmed - Seirafianpour F, Sodagar S, Pour Mohammad A, Panahi P, Mozafarpoor S, Almasi S, Goodarzi A. Cutaneous manifestations and considerations in COVID-19 pandemic: A systematic review. Dermatol Ther. 2020;33(6):e13986.
doi - Dowdle TS, Brown TK, Peterson JA, Banafshay K, Nguyen JM, Sturgeon ALE. COVID toes: A unique cutaneous indicator of COVID-19. The Southwest Respiratory and Critical Care Chronicles. 2021;9(39):15-21. Accessed Nov 11, 2021.
doi - Kolivras A, Dehavay F, Delplace D, Feoli F, Meiers I, Milone L, Olemans C, et al. Coronavirus (COVID-19) infection-induced chilblains: A case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489-492.
doi pubmed - Andina D, Noguera-Morel L, Bascuas-Arribas M, Gaitero-Tristan J, Alonso-Cadenas JA, Escalada-Pellitero S, Hernandez-Martin A, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37(3):406-411.
doi pubmed - Gallizzi R, Sutera D, Spagnolo A, Bagnato AM, Cannavo SP, Grasso L, Guarneri C, et al. Management of pernio-like cutaneous manifestations in children during the outbreak of COVID-19. Dermatol Ther. 2020;33(6):e14312.
doi pubmed - Hosseini-Tabatabaei A. Nails of the fingers and toes. Encyclopedia of Toxicology. 3rd Ed. 2014; p. 429-431. Accessed Nov 11, 2021.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
