| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Case Report
Volume 9, Number 3, September 2020, pages 92-97
Severe Cardiac and Abdominal Manifestations Without Lung Involvement in a Child With COVID-19
Miriam Gutierrez-Jimenoa, c, Adriana Ibanez Sadaa, Juan J Gaviraa, Carolina Cebrian-Nebota, Laura Martin Lopeza, Maria Macias Mojona, Marcos Garcia Howardb, Valentin Alzina de Aguilara
aUniversity of Navarra Clinic, Pamplona, Navarra, Spain
bComplejo Hospitalario de Navarra, Pamplona, Navarra, Spain
cCorresponding Author: Miriam Gutierrez-Jimeno, Department of Pediatrics, University of Navarra Clinic, Av. Pio XII, 36, 31008, Pamplona, Navarra, Spain
Manuscript submitted June 25, 2020, accepted July 7, 2020, published online July 30, 2020
Short title: Cardiac and Abdominal Symptoms of COVID-19
doi: https://doi.org/10.14740/ijcp387
| Abstract | ▴Top |
Coronavirus disease 2019 (COVID-19) has become a worldwide pandemic, affecting humans of all ages. Clinical features of the pediatric population have been published, but there is not yet enough information to make a definitive description. Fever is typical, as it is respiratory symptom. Rarely are the infection and complications severe, and, when they are, it is almost always in a patient with another underlying disease. However, some otherwise healthy children with COVID-19 do suffer critical organ injury, such as acute myocarditis, heart failure and gastrointestinal inflammation. The mechanism of these organ damages remains unclear. An otherwise normally healthy 13-year-old male was admitted to the pediatric intensive care unit with acute abdomen pain, possible myocarditis and a suspected diagnosis of COVID-19. Noteworthy basal findings were ventricular extrasystoles in the electrocardiogram (EKG) and moderate left ventricular systolic dysfunction. Chest X-ray was normal. Blood tests revealed altered levels of inflammation factors (C-reactive protein (CRP), D-dimer, fibrinogen, interleukin 6 (IL-6)), lymphopenia and elevated cardiac enzymes. The first test for polymerase chain reaction (PCR) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was negative. The patient’s condition worsened, and he entered cardiogenic shock (hypotension, tachycardia and oliguria). He was vomiting continuously, which made pain control difficult; imaging of his abdomen was undertaken. There was no response to fluid resuscitation, and so milrinone and epinephrine were administered. Empiric treatment began with azithromycin, foscarnet, carnitine and immunoglobulins. Hydroxychloroquine was given before the results of repeated SARS-CoV-2 and serology tests were available. Tocilizumab was administered once COVID-19 had been confirmed and massive inflammation had been observed. Progressively the clinical situation and the levels of the parameters studied improved. The patient was discharged 8 days after admission. Most children with SARS-CoV-2 infection are asymptomatic or present only mild symptoms. However, physicians should be aware of atypical and severe manifestations that may occur in the hyperinflammatory phase of the illness.
Keywords: Coronavirus; Myocarditis; Cardiogenic shock; Hyperinflammation; Colitis
| Introduction | ▴Top |
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent of coronavirus disease 2019 (COVID-19), apparently started in China in December 2019 with a cluster of unexplained cases of pneumonia [1]. Since then, it has rapidly spread to all continents, initially in Europe hitting hard in Italy and Spain [2].
COVID-19 is typically associated with dyspnea, fever, cough, myalgia and/or fatigue. Complications, such as acute respiratory distress syndrome (ARDS), acute cardiac injury and secondary infection have been described in adults [3, 4].
Despite the worldwide spread of COVID-19, children only represent around 1-2% of cases. Also, children seem to have milder clinical symptoms or are even asymptomatic. Common clinical features include fever, rhinitis, cough, fatigue, headache and diarrhea but more severe cases may occur with dyspnea, cyanosis and poor feeding [1].
We describe the case of an adolescent boy who was COVID-19 positive but had no flu-like symptoms and who developed cardiogenic shock and severe intestinal inflammation.
| Case Report | ▴Top |
A 13-year-old male was brought to the emergency department on April 21, 2020, with high fever that had started 2 days ago, abdominal pain, vomits and chest tightness. He stated that he did not have and had not had dyspnea or other respiratory symptoms. The boy’s medical history was unexceptional, and all vaccinations were up to date. Due to the country lockdown during the COVID-19 pandemic the patient was not attending school since March 16, 2020. He was of gypsy ancestry. No known family employment history. The patient was not under any medication prior to the diagnosis. The study has been approved and reviewed by the local ethic committee.
The boy was conscious and alert, no neck stiffness detected, pupils were isochoric and normally reactive. No neurological alarm symptoms. The patient complained about abdominal pain on palpation. Physical examination found fever (40 °C), mild dehydration, blood pressure of 90/60 mm Hg, tachycardia (140 bpm), oxygen saturation of 98% (breathing ambient air), and rashes on the palms of his hands and the soles of his feet (Fig. 1). Cardiopulmonary auscultation revealed only a gallop rhythm. Lung examination was normal. Electrocardiogram (EKG) findings were ventricular extrasystoles, minimal ST-segment elevation and T-wave inversion in lead V1 (Fig. 2). Chest radiography was normal (Fig. 3). Echocardiography found no structural heart anomalies and no alterations in coronary arteries but there was moderate left ventricular systolic dysfunction (left ventricular function evaluation (LVFE): 45%). Blood test counts and levels (with normal ranges given in parentheses) were as follows: lymphocytes 390 × 109/L (1,200 - 4,000 × 109/L), C-reactive protein (CRP) 13.88 mg/dL (< 0.5 mg/dL), procalcitonin (PCT) 0.86 ng/dL (> 0.5 ng/dL), interleukin 6 (IL-6) 558 pg/mL (< 7 pg/mL), D-dimer 1,810 ng/dL (150 - 500 ng/dL), fibrinogen 473 mg/dL (150 - 350 mg/dL), ferritin 329.6 ng/mL (30 - 400 ng/mL), troponin T 112.7 ng/L (< 14 ng/L), and pro b-type natriuretic peptide (pro-BNP) 4,051 pg/mL (< 194 pg/mL). Urinalysis is normal. Liver function: aspartate transaminase (AST) 39 U/L (1 - 40 U/L), alanine transaminase (ALT) 41 U/L (≤ 41 U/L), bilirubin total 0.77 mg/dL (≤ 1.2 mg/dL), gamma-glutamyl transferase (GGT) 21 U/L (≤ 60 U/L). Renal function: urea 19 mg/dL (16.6 - 48.5 mg/dL), creatinine 1.1 mg/dL (0.57 - 0.87 mg/dL). Ions normal: magnesium (Mg) 1.7 mg/dL (1.7 - 2.2 mg/dL), sodium (Na) 136 mEq/L (136 - 145 mEq/L), potassium (K) 4.5 mEq/L (3.5 - 5 mEq/L), chloride 100 mEq/L (98 - 197 mEq/L), bicarbonate 22.6 mEq/L (22 - 29 mEq/L), calcium (Ca) 8.8 mg/dL (8.4 - 10.2 mg/dL). Fluid resuscitation was carried out in the emergency department, and antibiotic treatment was given intravenous (IV) (ceftriaxone 2 g per day for 6 days). Blood culture is negative. The fever abated 12 h after admission.
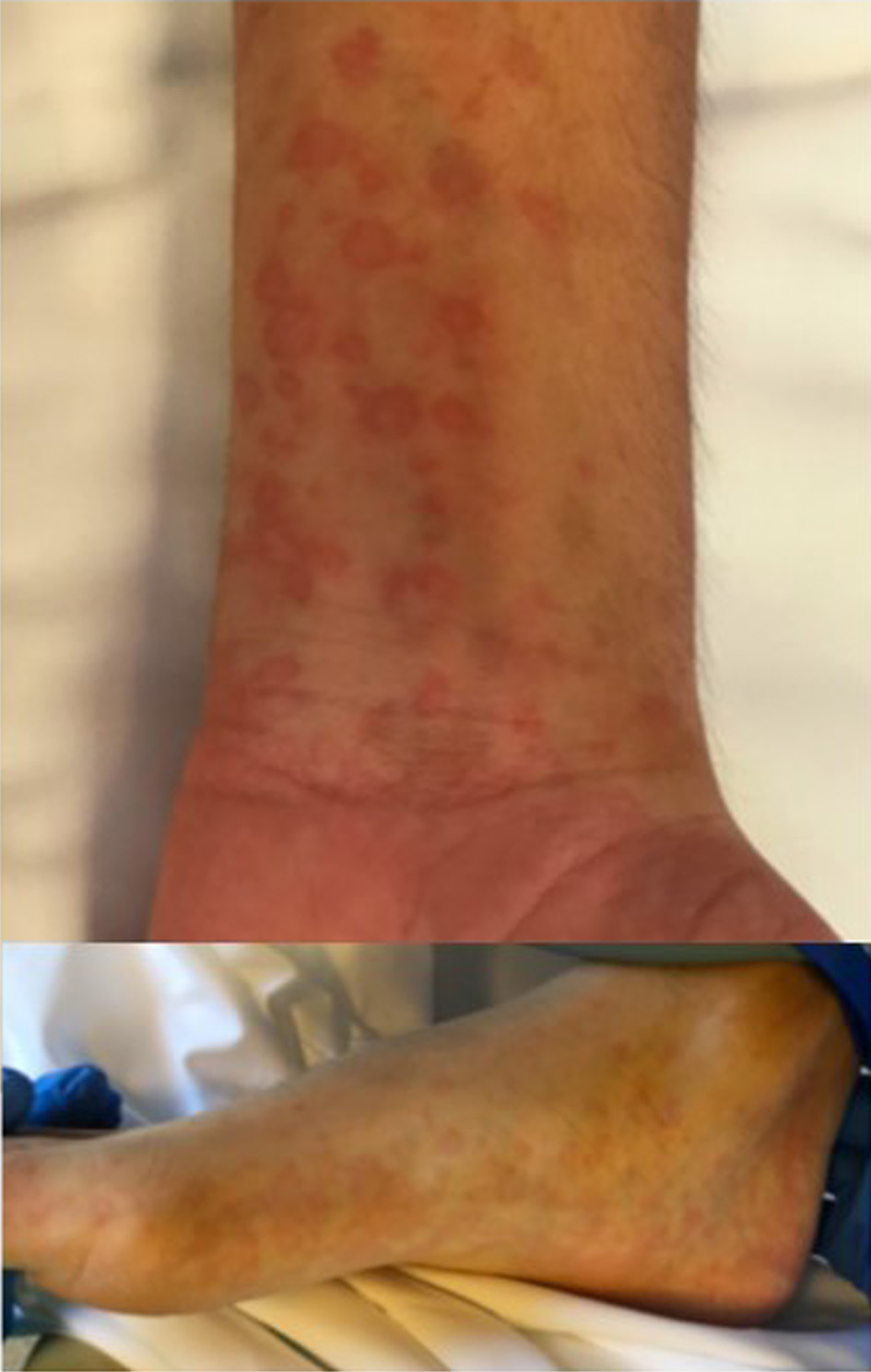 Click for large image | Figure 1. Erythematous annular lesions on palms and soles. They were not reported to itch or cause any pain, and they resolved without peeling or changes in skin pigmentation. |
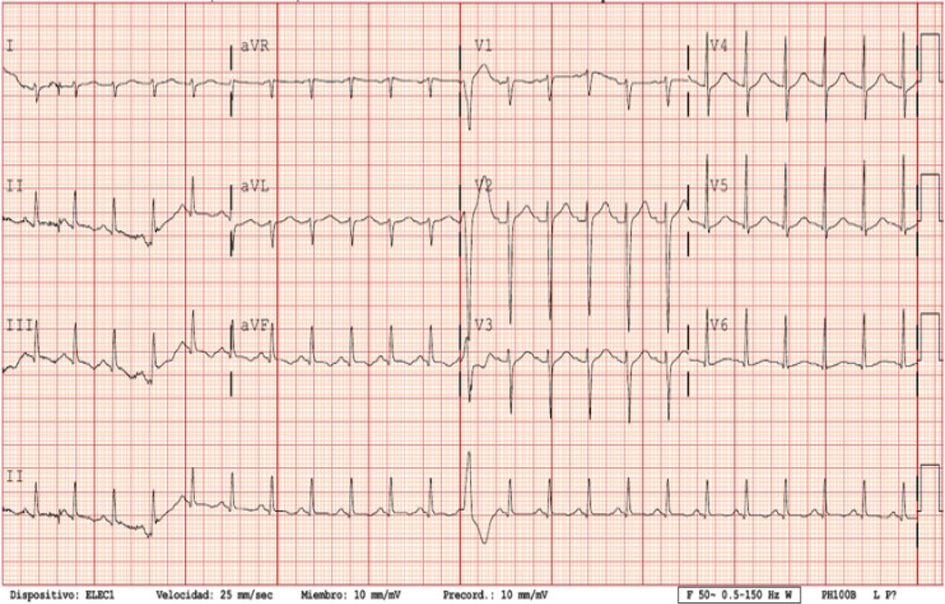 Click for large image | Figure 2. Electrocardiogram (EKG): 140 bpm, ventricular extrasystoles, minimal ST-segment elevation and T-wave inversion in lead V1. QT-c normal. |
 Click for large image | Figure 3. Chest X-ray: normal. It was repeated twice, and no signs suggested pneumonia. |
The patient was admitted to the intensive care unit (ICU) with a diagnosis of suspected myocarditis. Cardiac magnetic resonance imaging (MRI) (Fig. 4) evidenced generalized myocardial edema, T1 hyperintensity in the septal area and mild pericardial effusion. The patient was tested and found negative for the most frequent causes of myocarditis: adenovirus, parvovirus B19, cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus, human herpesvirus 6, varicella-zoster, Coxsackieviruses A and B, Influenza A and B viruses, respiratory syncytial virus (RSV), mycoplasma, toxoplasma and hepatitis A, B and C.
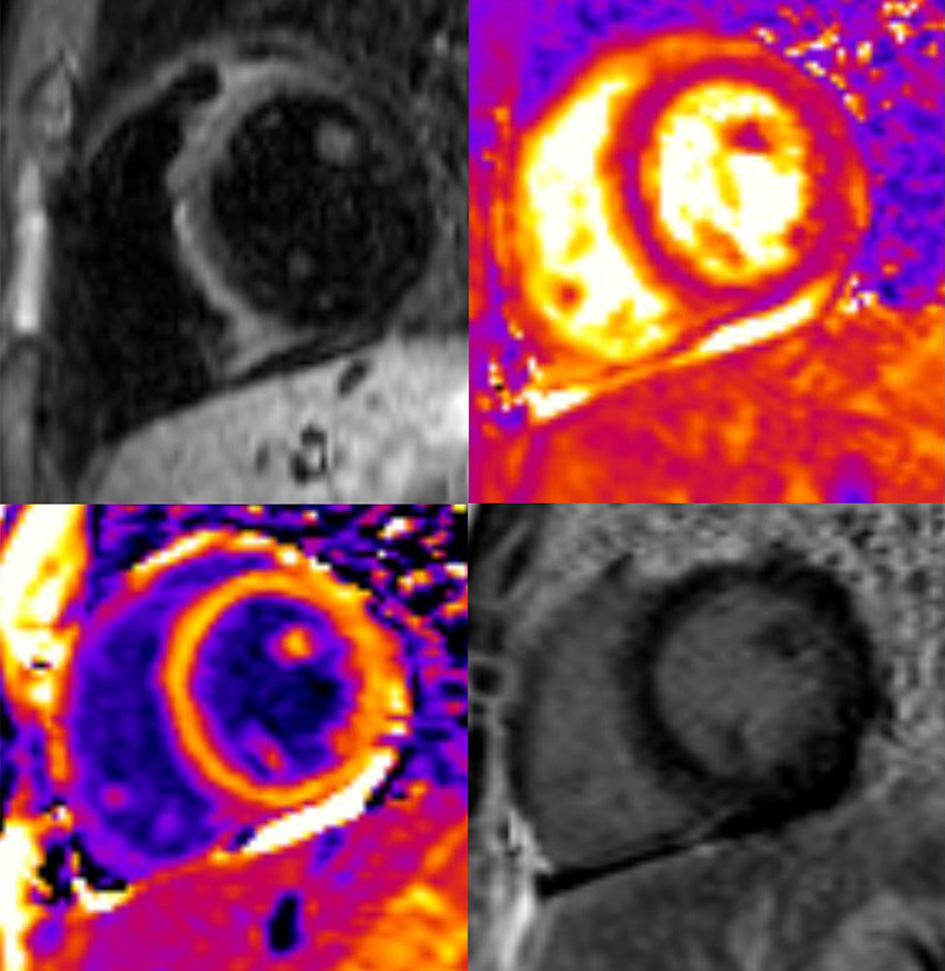 Click for large image | Figure 4. Cardiac MRI: generalized myocardial edema, T1 hyperintensity in the septal area and mild pericardial effusion. No late gadolinium enhancement was noted. MRI: magnetic resonance imaging. |
Empiric treatment was started with azithromycin via oral (10 mg/kg for 5 days), foscarnet IV (180 mg/kg/day for 2 days), carnitine via oral (1 g every 6 h for 2 days) and immunoglobulins (Igs) IV (1g/kg for 2 days). In view of the ongoing COVID-19 pandemic and because the patient lived with two family members (his mother and grandfather) who had tested positive for COVID-19, the patient was tested for SARS-CoV-2 three times by means of polymerase chain reaction (PCR) applied to samples obtained by nasopharyngeal swab. The first two test results were negative; the third was positive: a small quantity of SARS-CoV-2 ribonucleic acid (RNA) was detected. Additionally, serology tests found the patient to be IgG positive and IgM indeterminate for antibodies to coronavirus. Once SARS-CoV-2 was confirmed foscarnet and carnitine treatments were halted. Hydroxychloroquine via oral (400 mg every 12 h for the first 24 h and later 200 mg every 12 h for 4 days) and corticosteroids IV (2 mg/kg/day for 6 days) were administered. Tocilizumab was administered IV, in one dose, because D-dimer and CRP levels rose during the first 48 h. The IL-6 level also rose after the first determination (Table 1), and so low-molecular-weight heparin (LMWH) was introduced via subcutaneous (3,500 U) and maintained during hospitalization.
 Click to view | Table 1. Progression of Laboratory Test Values |
Abdominal pain became continuous, and vomiting, regular. The patient presented abdominal guarding. Abdominal ultrasound revealed a thickened appendix (7.2 mm) with destructuring of its layers, and the patient indicated rebound tenderness (positive sonographic Blumberg sign). These findings are compatible with incipient acute appendicitis or ileal inflammatory pathology, and so metronidazole IV (50 mg/kg/day for 7 days) was added to the previous antibiotic given in the emergency room (ceftriaxone). In view of the complexity of the case, abdominal computed tomography (CT) was done with the following results: hepatosplenomegaly, nonspecific thickening of the terminal ileum and segmental thickening of the ascending colon and transverse colon (nonspecific colitis) (Fig. 5). Surgical intervention was not indicated. Because of general oral intolerance, parenteral nutrition was started early and continued until day 4.
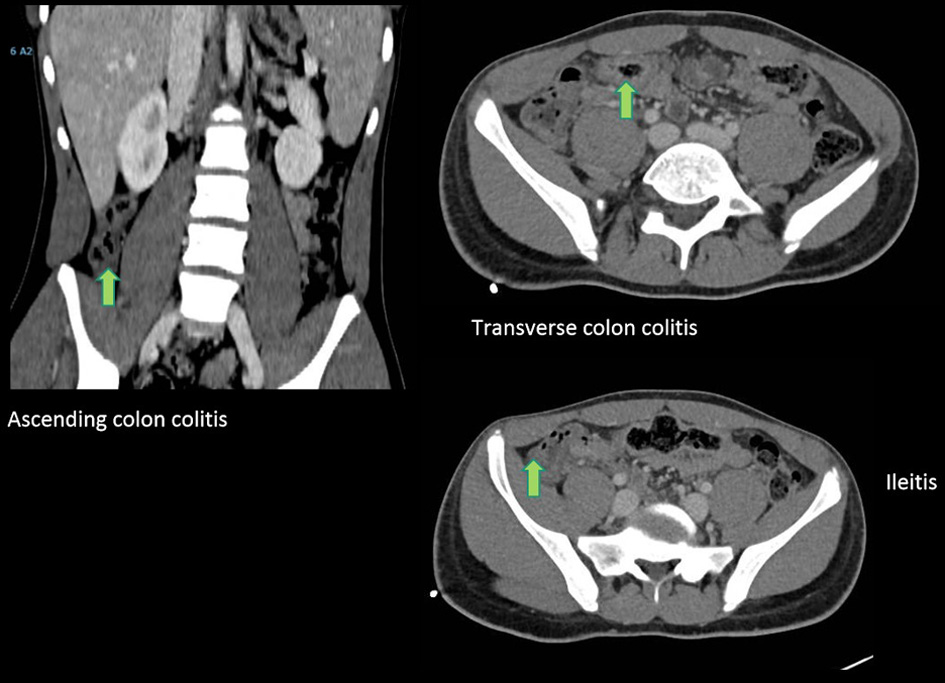 Click for large image | Figure 5. Abdominal CT: hepatosplenomegaly, nonspecific thickening of the terminal ileum (arrow) and segmental thickening of the ascending colon and transverse colon (arrows). CT: computed tomography. |
After ICU admission the patient remained hypotensive (with systolic blood pressure of 75 mm Hg and diastolic blood pressure of 35 mm Hg) and required inotropic support (milrinone 0.4 µg/kg/min and adrenaline 0.05 µg/kg/min). Cardiac function progressively stabilized over the first 3 days: levels of cardiac enzymes began to normalize (Table 1) and LVFE percentage improved. The patient was weaned off adrenaline on day 1 and off milrinone on day 3. Treatment directed at ameliorating the effects of heart failure was started on day 1 with daily doses via oral of furosemide (40 mg/day) and spironolactone (25 mg/day); once the inotropes had been withdrawn, carvedilol (6.25 mg/12 h) was introduced.
After 8 days in the ICU, the patient was discharged with normalized levels of cardiac enzymes and normal systolic function. The cutaneous lesions had resolved without peeling or changes in skin pigmentation. Under the treatment received, the patient’s recovery was satisfactory, with no noteworthy side effects, the results of all laboratory tests being completely normal.
| Discussion | ▴Top |
As of today, case reports and small series have described a presentation of acute illness accompanied by a hyper-inflammatory syndrome [5]. To our knowledge, when the patient was admitted no other similar cases had been published, hence the complexity of the case. However, it is fundamental to study and start empiric treatment taking into consideration the most common causes of myocarditis until a positive result is found by PCR or serology of SARS-CoV-2. We have also tried to report all aspects of the disease in children with all means available (CT, MRI, X-ray, laboratory tests and skin observation).
Most of the early clinical reports on COVID-19 focused on severe respiratory manifestations; as the pandemic spreads, however, new clinical features have been observed. In particular, myocardial injury, such as acute myocarditis, has been described in adult patients [6, 7], but few cases have been reported in children. It has been hypothesized that changes in heart tissue may be caused directly by SARS-CoV-2 replication through union with myocardial angiotensin converting enzyme 2 (ACE2) protein or by an excessive immune response [8].
In children, of all pathologies, acute viral myocarditis is the main cause of heart failure and can rapidly become life-threatening [9]. Early elucidation of the ethology allows pediatricians to use a target-treatment that improves prognosis. In the case reported here, we used both a general empiric treatment approach and drugs such as hydroxychloroquine, corticosteroids and tocilizumab [10]. (In passing, tocilizumab is an antibody against the IL-6 receptor, so it is reasonable to expect that, after infusion, free IL-6 would be higher, as occurred with our patient, whose IL-6 level rose by a factor of five).
Like IL-6, D-dimer, fibrinogen, and CRP are reliable biomarkers of inflammation. Heparin has anti-inflammatory properties and provides endothelial protection and may help to decrease cardiovascular damage in COVID-19 patients, with a consequent decrease of morbidity and mortality [11].
SARS-CoV-2 binds to gastrointestinal cells by means of the ACE2 receptor and the transmembrane protein serine protease 2. Subsequently, invasion and amplification of the virus triggers the so-called “COVID-19 cytokine storm” [12-14]. Digestive symptoms are common in COVID-19: diarrhea, nausea, vomiting, anorexia, and abdominal pain [15]. For most patients admitted with abdominal pain, chest images reveal disease effects in the base of the lungs [16, 17]. In contrast, in our patient CT imaging showed severe inflammation in the gastrointestinal tract, but MRI gave no evidence of disease in the lungs.
Other, recently-reported, extrapulmonary manifestations of COVID-19 include dermatological symptoms, such as, erythematous rash, widespread urticaria, varicella-like vesicles or dengue-like petechial rash [18, 19] but, to our knowledge, none similar to those observed in the patient reported here: from the first day of fever he had erythematous, non-pruritic palmoplantar and inguinal rashes, the individual lesions of which became annular before resolution.
SARS-CoV-2 RNA load in the nasopharynx is closely linked to illness severity in adults [20, 21]. In the case of our patient, SARS-CoV-2 RNA was only detected in the nasopharynx after two negative results. This could be the result of a possible high rate of false negatives associated with the test, poor sampling technique, low levels of the virus, or the absence of respiratory symptoms. Confronted by a negative result by nasopharyngeal swab/PCR, antibody-based techniques could be useful to better rule-out a diagnosis of COVID-19 [22]. SARS-CoV-2 has been found in stool samples up to 11 days after clearance of the virus from the respiratory tract [23, 24], and so the use of stool samples could be another approach to clarify diagnosis in cases with negative PCR, unclear serology and/or absence of respiratory symptoms.
In the case reported, we suspected that the patient was in the inflammatory phase of COVID-19 illness as opposed to the acute phase. The bases for this suspicion were that the patient was IgG positive; had a small quantity of SARS-CoV-2 RNA in the nasopharyngeal swab sample; and, finally, had cardiac MRI findings and abdominal CT findings indicative of inflammation rather than infection [25]. Cardiogenic shock and severe intestinal inflammation are possible emerging atypical presentations of COVID-19 in children.
Acknowledgments
We would like to sincerely thank Dr. Bastarrika for helping us with radiology imaging. We especially want to acknowledge Dr. Alzina’s whole life’s work and effort supporting his fellow colleagues.
Financial Disclosure
All the authors have no financial relationships relevant to this article to disclose.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
The informed consent statement has been obtained.
Author Contributions
Drs Gutierrez-Jimeno, Ibanez Sada, Cebrian-Nebot, Martin Lopez, Macias Mojon and Garcia Howard drafted the initial manuscript, collected data, reviewed and revised the manuscript. Dr Alzina and Dr Gavira conceptualized and designed the study, drafted the initial manuscript, supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors approved the final version to be published.
Data Availability
The authors declare that data supporting the findings of the study are available within the article.
| References | ▴Top |
- Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(5):355-368.
doi pubmed - Saglietto A, D'Ascenzo F, Zoccai GB, De Ferrari GM. COVID-19 in Europe: the Italian lesson. Lancet. 2020;395(10230):1110-1111.
doi - Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
doi - Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207.
doi pubmed - Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607-1608.
doi - Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, Wang LF, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020.
doi pubmed - Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41(19):1859.
doi pubmed - Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, Cheng Y, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116-121.
doi pubmed - Tunuguntla H, Jeewa A, Denfield SW. Acute myocarditis and pericarditis in children. Pediatr Rev. 2019;40(1):14-25.
doi pubmed - Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954.
doi pubmed - Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18(5):1020-1022.
doi pubmed - Zhang H, Li HB, Lyu JR, Lei XM, Li W, Wu G, Lyu J, et al. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis. 2020;96:19-24.
doi pubmed - Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8.
doi pubmed - Effenberger M, Grabherr F, Mayr L, Schwaerzler J, Nairz M, Seifert M, Hilbe R, et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020;69(8):1543-1544.
doi pubmed - Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35(5):744-748.
doi pubmed - Siegel A, Chang PJ, Jarou ZJ, Paushter DM, Harmath CB, Arevalo JB, Dachman A. Lung Base Findings of Coronavirus Disease (COVID-19) on Abdominal CT in Patients With Predominant Gastrointestinal Symptoms. AJR Am J Roentgenol. 2020:1-3.
doi pubmed - Poggiali E, Ramos PM, Bastoni D, Vercelli A, Magnacavallo A. Abdominal Pain: A Real Challenge in Novel COVID-19 Infection. Eur J Case Rep Intern Med. 2020;7(4):001632.
doi pubmed - Marzano AV, Genovese G, Fabbrocini G, Pigatto P, Monfrecola G, Piraccini BM, Veraldi S, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280-285.
doi pubmed - Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212-e213.
doi pubmed - Liu Y, Liao W, Wan L, Xiang T, Zhang W. Correlation between relative nasopharyngeal virus RNA load and lymphocyte count disease severity in patients with COVID-19. Viral Immunol. 2020.
doi pubmed - Romera-Liebana L, Orfila F, Segura JM, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. MedRxic. 2020.
- Xiang F, Wang X, He X, Peng Z, Yang B, Zhang J, Zhou Q, et al. Antibody detection and dynamic characteristics in patients with COVID-19. Clin Infect Dis. 2020.
- Wan Y, Shang J, Graham R, et al. COVID-19, the gastrointestinal tract: more than meets the eye. J Virol. 2020;94(7):1-2.
- Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502-505.
doi pubmed - Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405-407.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
