| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Case Report
Volume 9, Number 3, September 2020, pages 77-81
Mycoplasma pneumoniae Infection in a 13-Year-Old Girl With Down’s Syndrome During the COVID-19 Pandemic
Tiphaine Corbisiera, f, Olga Chatzisb, Dimitri Van Der Lindenb, Pierre-Florent Petitc, David Tuerlinckxd, Dana Dumitriue, Astrid Haenecoura
aPediatric Intensive Care Unit, Cliniques Universitaires Saint-Luc, UCLouvain, Brussels, Belgium
bPediatric Infectious Diseases, Department of Paediatrics, Cliniques Universitaires Saint-Luc, UCLouvain, Brussels, Belgium
cDe Duve Institute, Ludwig Institute for Cancer Research, Universite Catholique de Louvain, Brussels, Belgium
dCHU Dinant-Godinne, Department of Paediatrics, Universite Catholique de Louvain, Yvoir, Belgium
eRadiology Department, Cliniques Universitaires Saint-Luc, Universite Catholique de Louvain, Brussels, Belgium
fCorresponding Author: Tiphaine Corbisier, Cliniques Universitaires Saint-Luc, Avenue Hippocrate 10, 1200 Bruxelles, UCLouvain, Brussels, Belgium
Manuscript submitted June 8, 2020, accepted June 23, 2020, published online July 30, 2020
Short title: Mycoplasma pneumoniae Infection
doi: https://doi.org/10.14740/ijcp384
| Abstract | ▴Top |
We report the case of a 13-year-old girl with Down’s syndrome who presented with cough, fever, and a rapidly evolving respiratory failure leading to intensive care unit transfer during coronavirus disease 2019 (COVID-19) outbreak in Belgium. COVID-19-related acute respiratory distress syndrome (ARDS) is rare in teenagers and its diagnosis remains challenging as reverse transcription-polymerase chain reaction (RT-PCR)-testing sensitivity and radiological criteria still have to be defined in that population. We finally concluded to severe Mycoplasma pneumoniae infection. This case report gives the opportunity to discuss the rare occurrence of that disease, and to review the radiological findings of M. pneumoniae- and COVID-19-related pneumonia in teenagers.
Keywords: ARDS; SARS-CoV-2; Mycoplasma pneumoniae
| Introduction | ▴Top |
Pneumonia stays one of the most common reasons for hospitalization in children, with an overall incidence rates of 14.4 per 10,000 children aged 0 - 16 years per year [1]. Bilateral pneumonia leading to non-invasive ventilation is rare in teenagers. The causative pathogens are mainly viruses and some bacteria, mostly Mycoplasma pneumoniae. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged in December 2019 in China as a new virus responsible for acute respiratory distress syndrome (ARDS) and it has spread worldwide since then [2]. Although it has been quite early identified that children were rarely affected compared to adults, SARS-CoV-2 pandemic disrupted the classical management of children with pneumonia in pediatric intensive care unit (PICU). We report the case of a teenager who presented with bilateral pneumonia during coronavirus disease 2019 (COVID-19) outbreak in Belgium and for which COVID-19 suspicion strongly impacted both diagnostic hypothesis and treatment plan.
| Case Report | ▴Top |
A 13-year-old girl with Down’s syndrome (DS) presented to the emergency department on April 2, 2020 with a 7-day history of dry cough and high fever. She had no gastrointestinal symptoms or olfactory disorders. She received symptomatic treatment at home comprising mucolytic syrup (containing carbocisteine) and acetaminophen. On physical examination her temperature was 38.3°C, heart rate was 98/min, respiratory rate was 40/min with a paradoxical breathing, and her blood pressure was 146/80 mm Hg. Upon cardiac auscultation, the rhythm was rapid but regular, no cardiac murmur was detected. Capillary refill time was normal. Pulmonary auscultation was abnormal with bilateral reduced air entry and symmetrical basal crackles. She had intercostal and subcostal retractions. Meningeal signs were absent, but consciousness state was variable with both agitation and drowsiness episodes. She had no skin eruption but a bilateral dry conjunctivitis. She presented typical physical features of DS such as upslanting palpebral fissures, flat nasal bridge, low set of ears, epicanthal folds. The rest of clinical examination was unremarkable. The oxygen saturation was 86% without oxygen support and rose to 95% with a non-rebreather mask. A trial of inhaled bronchodilators (salbutamol) did not result in any clinical improvement.
She was born at term, with a birth weight of 2.6 kg (P3 - 10). She was the fourth child of a couple of healthy parents. There was no history of consanguinity marriage of their parents. DS was diagnosed at birth. She was hospitalized for an oxygen-requiring respiratory syncytial virus bronchiolitis at the age of 3 months and for a probable viral bronchopneumonia of the right upper lobe at the age of 11 months. At that time, skin testing with prick technique showed a sensitization for cat.
She underwent a patch closure of a ventricular septal defect, and atrial septal defect and a patent ductus ligation at 12 months of age. She had a two-step cataract surgical treatment at 15 and 16 months. Developmental physiotherapy had been established since the neonatal period. At the age of 2 years, she took her first steps and said three words. She developed attention and speech disorders and integrated an adapted education program. She gradually caught up the weight and height curves. At the age of 4 years, she was at P70 for the weight and P95 for the height. She had been tested and found negative for hypothyroidism.
Her parents were working in the laundry service of a nursing home affected by SARS-CoV-2 outbreak, but they did not report any symptom of respiratory infection. There was no pet at home. She was in order for vaccination according to the Belgian calendar. She did not receive flu vaccination this winter.
Arterial blood gases analysis upon ambient air showed hypoxemia (partial pressure of arterial oxygen (PaO2): 68 mm Hg), and a respiratory alkalosis. Laboratory investigations showed an increased C-reactive protein level (Table 1), a normal white blood cell count (WBC) of 6,219/mm3 and a lymphopenia (669/mm3). Blood cultures were collected by the central venous catheter (Bactec BD™, 5 days of incubation).
 Click to view | Table 1. Laboratory Findings of the Patient |
Chest X-ray showed bilateral pulmonary parenchymal infiltrates, leading to the performance of a chest computed tomography (CT). Although the chest CT had a degree of movement artifact, it showed some bilateral ground glass opacities of peripheral distribution involving mostly the inferior left lobe. There were bronchial walls thickening, mediastinal lymphadenopathy and pleural effusion (Fig. 1). The chest CT findings were considered compatible with, but not typical for COVID-19 pneumonia.
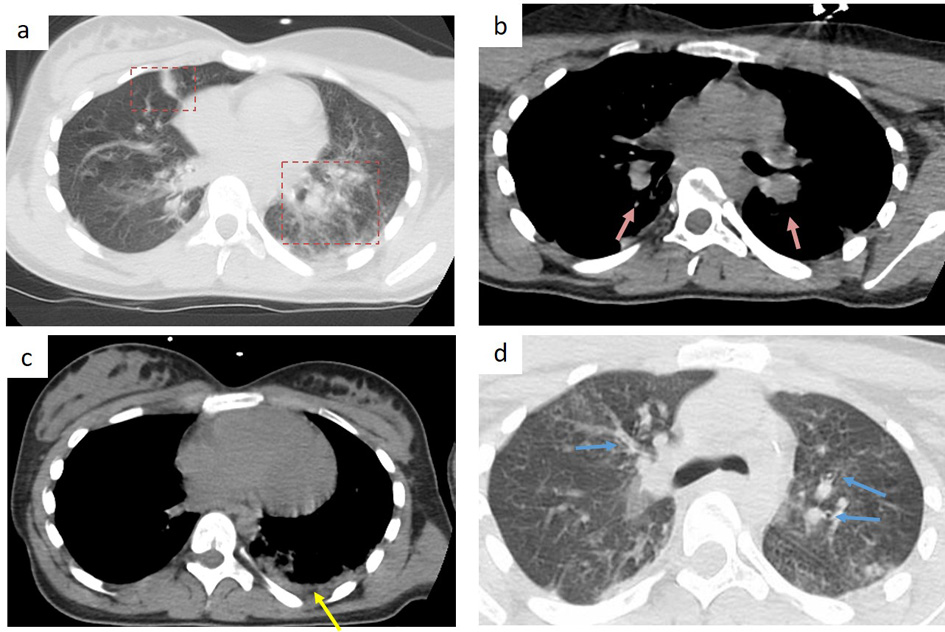 Click for large image | Figure 1. Chest CT findings in a 13-year-old girl, 7 days after symptoms’ onset. (a) bilateral peripheral patchy ground-glass opacity (red frame), predominant in the left inferior lobe; (b) bilateral hilar lymphadenopathy (pink arrow); (c) pleural effusion (yellow arrow); (d) bronchial wall thickening (blue arrow). |
A SARS-CoV-2-specific real-time polymerase chain reaction (PCR) assay (Primerdesign Ltd, Chandler’s Food, UK), targeting the ribonucleic acid (RNA)-dependent RNA polymerase (RdRp) gene was performed on a nasopharynx swab and she received one dose of 6.5 mg/kg (300 mg) of oral hydroxychloroquine. From that point, all caregivers have worn personal protective equipment (PPE).
Considering the severe pneumonia with hypoxemia, she also received 1.6 g intravenous (IV) of amoxicillin-clavulanic acid in the emergency room. The treatment was however discontinued after the second dose as the radiological and biological findings were not in favor of a bacterial pneumonia. During the hospitalization, she received orally lorazepam for agitation control for 5 days (maximal dose: 0.1 µg/kg).
She was admitted to our PICU in a negative pressure room for ARDS requiring non-invasive ventilation (NIV) (PaO2/fraction of inspired oxygen (FiO2) ratio of 69 mm Hg).
She was also tested for M. pneumoniae (PCR on nasopharynx swab and blood serology), Legionella pneumoniae and Streptococcus pneumoniae (urine antigen detection, immunochromatography BinaxNOW™ Alere).
A cardiac ultrasound was performed and showed a corrected heart anatomy with a normal function. She presented a prolonged corrected QT segment (480 ms) on her admission electrocardiogram (ECG).
The first analysis of nasal swab came back negative for SARS-CoV-2. As the clinical state showed no improvement, she was retested 48h after the first test. The second reverse transcription (RT)-PCR testing was again negative for COVID-19. L. pneumoniae and S. pneumonia antigens were not detected in urine. Blood cultures were negative. M. pneumoniae PCR on nasal swab was positive (Argene® respiratory kit). She received oral azithromycin (from day 6 of PICU stay) at a dose of 500 mg on the first day and 250 mg for 4 days.
She gradually improved and was discharged from PICU after 13 days. She remained hemodynamically stable and did not show any gastro-intestinal manifestations. In total, the patient received NIV for 13 days (bilevel positive airway pressure (BiPAP) for 8 days and continuous positive airway pressure (CPAP) for 5 days), with an initial hypoxic state requiring a FiO2 of 1, gradually evolving to reach 0.25 at day 12 of NIV. After her PICU stay, the patient was hospitalized for 4 days in a general pediatric service and was progressively weaned off oxygen. She came back home without any symptoms.
Antibody testing for M. pneumoniae on serum after 8 days of symptoms showed positive immunoglobin M (IgM) and negative immunoglobin G (IgG) levels, and a control test at 15 days’ interval showed raising IgG level (99.6 AU/mL, negative < 10 AU/mL) with positive IgM, supporting the hypothesis of a recent infection. Twenty days after onset of symptoms, COVID-19 serology became available and was tested on serum but neither IgM nor IgG (by an enzyme-linked immunosorbent assay (ELISA)) were detected.
| Discussion | ▴Top |
In this case report, we describe the management of a teenager with DS presenting fever, bilateral pneumonia and hypoxemia. Severe infectious pneumonia with bilateral infiltrates requiring NIV is rare in that population and considering SARS-CoV-2 pandemic in Belgium, COVID-19-related pneumonia was suspected. We concluded that the pneumonia was actually related to M. pneumoniae, although the severe clinical presentation was unusual for that pathogen. The management was particularly challenging as COVID-19 suspicion modulated therapeutic management and warranted the setting of strong sanitary measures.
During the SARS-CoV-2 pandemic, the diagnosis of COVID-19 can be suspected in children but is actually rarely confirmed. From the current and recent knowledge, children seem to be less susceptible to COVID-19 [2]. Symptomatic infection appears to be relatively uncommon under the age of 20 years (2%), although some severe cases have been reported [2, 3]. Specific pediatric clinical and radiological features of COVID-19 pneumonia have therefore not yet been clearly identified [4].
RT-PCR on nasopharynx swab has been validated in adults, with a low sensitivity (56-83%) at early stage of the disease [5]. Sensitivity and specificity of the RT-PCR have not yet been evaluated in children. Chest CT offers high sensitivity, but moderate specificity for COVID-19 pneumonia in adults. COVID-19 pneumonia is more likely to have peripheral distribution (80% vs. 57%), ground-glass opacity (91% vs. 68%), fine reticular opacity (56% vs. 22%), vascular thickening (59% vs. 22%) and reverse halo sign (11% vs. 1%) compared to non COVID-19 (viral or bacterial) pneumonia [6]. Pediatric patients with COVID-19 pneumonia tend to have lower rate of positive chest-CT findings than adults [7]. They have milder symptoms and therefore have a higher prevalence of negative chest-CT, as well as less extensive abnormalities on imaging [7]. Peribronchial distribution and bronchial wall thickening are more commonly seen in pediatric patients than adults [7]. Hence, chest CT is currently not an appropriate diagnosis tool for COVID-19 in children.
For all these reasons, diagnosis of COVID-19 in children is challenging. In our reported case, COVID-19 was highly suspected according to epidemic spreading in Belgium at that time, the contact exposure of both parents, a convincing clinical history and chest-CT findings. COVID-19 was not detected by RT-PCR, but isolation was maintained, given the low sensitivity of the testing and paucity of data in children. Hydroxychloroquine treatment was discontinued after the first dose according to the absence of positive COVID-19 result, the unclear benefit of hydroxychloroquine in COVID-19 infection and the presence of a prolonged QT.
The differential diagnosis of acute respiratory distress in children included other viral pulmonary infections and atypical bacterial pneumonia due to L. or M. pneumoniae. Influenza was not tested as the epidemic period was already over in Belgium. L. pneumoniae urinary antigen test was negative, and the good clinical response to azithromycin alone was not favoring this hypothesis. The second nasal swab for COVID-19 testing remained negative. The result was considered with caution due to the fact that it was collected 10 days after the onset of symptoms making the probability of a positive test possibly low [8]. In addition, it has been shown that RT-PCR testing on lower respiratory tract specimens displayed better sensitivity, but clinical evolution under NIV did not warrant invasive procedure such as bronchoalveolar lavage [9].
Antibody testing (chemiluminescent immunoassay, Snibe Diagnostic) was performed 20 days after the first symptoms definitely ruled out the hypothesis of a SARS-CoV-2 infection in our patient (95.60% of sensitivity, 96% of specificity). Of note, lymphopenia is a common finding in COVID-19 but it is also very common in patients with DS [10].
M. pneumoniae is one of the common pathogens leading to community-acquired pneumonia. It can occur at any age, but adolescents are predominantly affected [11]. The rate of hospitalization in PICU is quite low, but some severe cases are described [11, 12]. Viral co-infection is known to be frequent (27%) with M. pneumoniae and the hypothesis of a co-infection with COVID-19 was not unlikely [11, 13]. The positivity of M. pneumoniae IgM in the acute stage, the detection of the bacterial antigen by PCR in nasopharyngeal swab, and the further increase in M. pneumonia Ig G titer 2 weeks later confirmed the ongoing infection.
Radiographic findings of M. pneumonia are non-specific but may include consolidation, single or multi-lobar infiltrates, unilateral or bilateral infiltrates, pleural effusion and hilar adenopathy. Chest CT is rarely performed but is more sensitive and mostly shows airspace consolidation or ground-glass opacities [14].
Patients with DS are highly prone to respiratory tract viral and bacterial infections, and these respiratory infections are more severe than in the general pediatric population. These patients have abnormal upper airway physiology, and they are prone to asthma [15]. They otherwise present immune dysregulation in both innate and adaptive immune system. WBCs are known to be decreased in DS patients [10].
Suspicion for COVID-19 tremendously complicated this patient’s stay in PICU. The patient was isolated in a negative pressure room; caregivers wore the complete PPE, including filtering facepiece 2 (FFP2) mask, eye protection, gloves, hat and coat. Those procedures were obviously impairing communication especially with a young patient with DS, already known for having facial recognition difficulties. Limited number of healthcare workers in the room and restricted parental visits leaded to loneliness, which can trigger agitation, decrease tolerance to NIV and finally increase the need for sedation. This case illustrates the complexity of implementation of strict infection control measures into PICUs, which are already very unfamiliar environments for pediatric patients under normal circumstances.
Conclusions
This case highlights the challenges in the management of pneumonia in children during COVID-19 pandemic, as it introduces doubts in the differential diagnosis and strictly restrains the contacts with caregivers.
This 13-year-old girl with DS presented clinical, biological and radiological findings suggesting COVID-19. She was finally diagnosed with severe M. pneumoniae infection requiring NIV. Reliability of biological tests for SARS-CoV-2 detection in children still needs to be further evaluated. Typical radiological findings of COVID-19 pneumonia in children have been established on small cohorts and require further validation.
Acknowledgments
We acknowledged the patient and her family for consenting for the information and images to be published.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The family consent has been obtained in writing.
Author Contributions
All authors provided the clinical case concept and reviewed the final manuscript.
Data Availability
The authors declare that data supporting the findings of this case report are available within the article.
| References | ▴Top |
- Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, Thomson A, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl 2):ii1-23.
doi pubmed - CDC COVID-19 Response Team. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422-426.
doi pubmed - Ong JSM, Tosoni A, Kim Y, Kissoon N, Murthy S. Coronavirus disease 2019 in critically ill children: a narrative review of the literature. Pediatr Crit Care Med. 2020.
doi pubmed - Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020.
doi pubmed - Kokkinakis I, Selby K, Favrat B, Genton B, Cornuz J. [Covid-19 diagnosis : clinical recommendations and performance of nasopharyngeal swab-PCR]. Rev Med Suisse. 2020;16(689):699-701.
- Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, Pan I, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020:200823.
doi pubmed - Chen A, Huang J, Liao Y, et al. Differences in clinical and imaging presentation of pediatric patients with COVID-19 in comparison with adults. Radiol Cardiothorac Imaging. 2020;2(2):e200117.
doi - Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177-1179.
doi pubmed - Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020.
doi - Roizen NJ, Amarose AP. Hematologic abnormalities in children with Down syndrome. Am J Med Genet. 1993;46(5):510-512.
doi pubmed - Moynihan KM, Barlow A, Nourse C, Heney C, Schlebusch S, Schlapbach LJ. Severe mycoplasma pneumoniae infection in children admitted to pediatric intensive care. Pediatr Infect Dis J. 2018;37(12):e336-e338.
doi pubmed - Yoshida T, Asato Y, Kukita I, Ooshiro M, Takara I, Iha H, Sugahara K, et al. A 7-year-old boy with mycoplasmal infection requiring extracorporeal membrane oxygenation. Eur J Pediatr. 2003;162(1):44-46.
doi pubmed - Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020;55(5):1169-1174.
doi pubmed - Reittner P, Muller NL, Heyneman L, Johkoh T, Park JS, Lee KS, Honda O, et al. Mycoplasma pneumoniae pneumonia: radiographic and high-resolution CT features in 28 patients. AJR Am J Roentgenol. 2000;174(1):37-41.
doi pubmed - Watts R, Vyas H. An overview of respiratory problems in children with Down's syndrome. Arch Dis Child. 2013;98(10):812-817.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
