| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Case Report
Volume 9, Number 1, March 2020, pages 9-15
Continuous Positive Airway Pressure Belly Syndrome: Challenges of a Changing Paradigm
Archana Priyadarshia, b, d, Murray Hindera, Nadia Badawib, c, Melissa Luiga, Mark Tracya, b
aNeonatal Intensive Care Unit, Westmead Hospital, Sydney, NSW, Australia
bDepartment of Pediatrics and Child Health, Sydney University, Sydney, NSW, Australia
cGrace Centre for Newborn Care, Sydney Children’s Hospital Network, Westmead, NSW, Australia
dCorresponding Author: Archana Priyadarshi, Neonatal Intensive Care Unit, Westmead Hospital, Sydney, NSW 2145, Australia
Manuscript submitted December 2, 2019, accepted December 23, 2019
Short title: CPAP Belly Syndrome
doi: https://doi.org/10.14740/ijcp352
| Abstract | ▴Top |
In extreme preterm infants, early use of continuous positive airway pressure (CPAP) for respiratory support reduces the incidence of chronic lung disease. However, as a sequel, inadvertent passage of air into the gastrointestinal tract leads to abdominal distension often with visibly dilated loops. The first description of “CPAP belly syndrome” in 1992, originates from the study in premature infants weighing less than 1,000 g and managed on nasal CPAP. The description of this phenomenon included benign episodic abdominal distension, with no associations to feed intolerance, and no radiological evidence of bowel wall thickening, pneumatosis or free air. With improving perinatal care, lesser gestational age infants are increasingly managed on early CPAP with resultant more frequent occurrence of CPAP belly syndrome. When extreme preterm infants on CPAP develop tense, marked abdominal distension, clinical decisions to cease feeds, administer empiric antibiotics and perform plain abdominal radiographs are all justified to screen for potentially serious causes. With rampant use of non-invasive respiratory support in extreme preterm infants, the occurrence of severe CPAP belly syndrome now extends to include clinical scenario mimicking a “necrotizing enterocolitis (NEC) scare”. We present the case of an extreme preterm infant with severe CPAP belly syndrome that required rescue intubation due to a massively distended abdomen. The emergency management included change to invasive ventilation and exclusion of serious intestinal conditions such as NEC. In retrospect, the life-threatening marked abdominal distension was due to severe CPAP belly syndrome, contrary to its well-recognized benign description, three decades ago. The clinical paradigm of CPAP belly syndrome is evolving, and in its severe form in extreme preterm infants, warrants vigilant monitoring to differentiate it from severe progressive intestinal conditions, such as NEC. Further research is required to describe its causes, associated morbidities and the need to evaluate the utility of other diagnostic modalities to reassure clinicians.
Keywords: CPAP belly syndrome; Abdominal distension; Nasal CPAP
| Introduction | ▴Top |
In extreme preterm infants, early proactive use of continuous positive airway pressure (CPAP) reduces the risk of lung injury and need for endotracheal intubation. The development of chronic lung disease (CLD) in these infants is strongly associated with the use of mechanical ventilation and thus, CPAP is the preferred form of respiratory support [1-3]. Devices for the delivery of CPAP vary amongst neonatal units, including: 1) bubble CPAP, 2) ventilator CPAP, and 3) infant flow driver [4]. Bubble CPAP is a safe and cost-effective option [5, 6] which is routinely used in our unit to provide continued respiratory support for preterm infants.
Gaseous abdominal distension associated with the use of nasal CPAP was first described by Jaile et al in 1992 [7] in their study of 25 premature infants weighing less than 1,000 g. They described this manifestation as “CPAP belly syndrome”. The description included the abdominal distension is benign not associated with feed intolerance, necrotizing enterocolitis (NEC) or bowel obstruction and occurs due to the use of nasal CPAP, aerophagia and immaturity of bowel motility in preterm infants. This phenomenon usually occurs 4 - 7 days after treatment with nasal CPAP, with development of soft, strikingly distended abdomen and visibly dilated bowel loops, not present at birth. They concluded that CPAP belly syndrome does not warrant cessation of feeding and there were no concerns over continuing CPAP [7].
Advances in respiratory support management have led to better-fitting nasal interfaces for the delivery of CPAP to improve pulmonary mechanisms. However, much is still not known about gastrointestinal gaseous distension and its complications. A typical scenario for a neonatal clinician managing extreme preterm infants on CPAP is the occurrence of marked abdominal distension, feed intolerance, with or without bile stained gastric aspirates. In our experience, the clinical presentation of CPAP belly syndrome in extreme preterm infants can be dramatic, mimicking acute NEC scare, necessitating urgent diagnostic evaluation.
The clinical diagnosis of CPAP belly syndrome relies on palpation of a soft, distended, non-tender abdomen along with plain abdominal radiograph demonstrating 1) distended abdominal cavity (cross-diameter); 2) almost symmetrical polygonal gas/air spaces spread homogenously with thinned out bowel walls, and presence of rectal gas; 3) non-thickened bowel wall; and 4) absence of pneumatosis intestinalis, portal venous gas and non-moving (stagnant) parallel sausage-shaped bowel loops. These findings may be reassuring, but in the context of feed intolerance and non-specific findings on plain abdominal radiograph, a decision to withhold feeds, halt increments in feed volume and use empiric antibiotics is justifiable and a frequent occurrence. Marked abdominal distension resulting in restriction of the diaphragm and reduced chest expansion is a severe complication requiring escalation of respiratory support to invasive ventilation. This complication reflects the changing paradigm of CPAP belly syndrome. We present an illustrative example below of an extreme preterm infant, identified as AJ, with severe CPAP belly syndrome.
Marked abdominal distension arising from severe CPAP belly syndrome can compromise breathing, as demonstrated in our case. While preventing lung injury with early use of CPAP in extreme preterm infants is clearly established, the clinical presentation of CPAP belly syndrome in such infants is evolving with its diagnostic and management challenges, necessating the need to redefine this changing paradigm.
| Case Report | ▴Top |
Our case, AJ, was born at 27 weeks gestation with a birth weight of 850 g (23rd percentile) with head circumference of 24 cm at birth (34th percentile) and length of 33 cm (30th percentile) with Apgar scores of 8, 9 at 1 and 5 min, respectively. Mother, a primigravidae, has history of bicornuate uterus. This pregnancy was complicated by the presence of intra-uterine growth restriction, clinical and placental histopathological evidence of chorioamnionitis, resulting in preterm delivery. Mother received 5 days of ampicillin and gentamicin intravenously prior to delivery. There was no history of consanguinity. Parents belonged to middle socio-economic status group and there was no other significant medical history.
AJ was hemodynamically stable at birth, however developed severe respiratory distress syndrome (RDS), requiring surfactant therapy and a brief period of ventilation in the immediate perinatal period. He was extubated to ventilator CPAP via nasal mask at 12 h of age. His respiratory status remained stable on ventilator CPAP pressure of 7 cm of H2O, with a flow of 6 L/min, and fraction of inspired oxygen (FiO2) of 25-30% (pulse oximetry targets of 89-94%). At 24 h of life, detection of a hemodynamically significant patent ductus arteriosus (PDA) on point-of-care cardiac ultrasound (US) led to medical treatment with intravenous ibuprofen, with closure detected on day 4. AJ received total parenteral nutrition (TPN) and expressed breast milk reaching full enteral feeds (150 mL/kg/day) on day 14 of life.
AJ remained stable on continued CPAP support and was transitioned to bubble CPAP of 7 cm H2O in 6 L/min of flow, at 3 weeks of age. Soon after this change, he required an increase in his CPAP to 8 cm of H2O due to work of breathing. On these CPAP settings, AJ developed gaseous abdominal distension, with visible bowel loops. At this stage, there were no clinical concerns with this new-onset abdominal distension. His abdomen was soft, non-tender, and bowel sounds were heard on auscultation regularly. However, as the abdominal distension persisted on CPAP of 8 cm of H2O, AJ had a screening chest and abdominal radiograph. The chest X-ray demonstrated no new lung field abnormalities compared to his previous films. The abdominal X-ray demonstrated homogenous dilatation of the bowel loops throughout, consistent with CPAP belly syndrome (Fig. 1). For the next 3 weeks, AJ remained stable on continued CPAP support with PCO2 ranging from 45 to 60 mm Hg, and normal pH on capillary blood gases. Frequent clinical examination and regular abdominal girth measurements were performed. During this episode, he continued to tolerate milk feeds of 170 mL/kg/day (expressed breast milk with added human milk fortifier to achieve 24 kcal/30 mL).
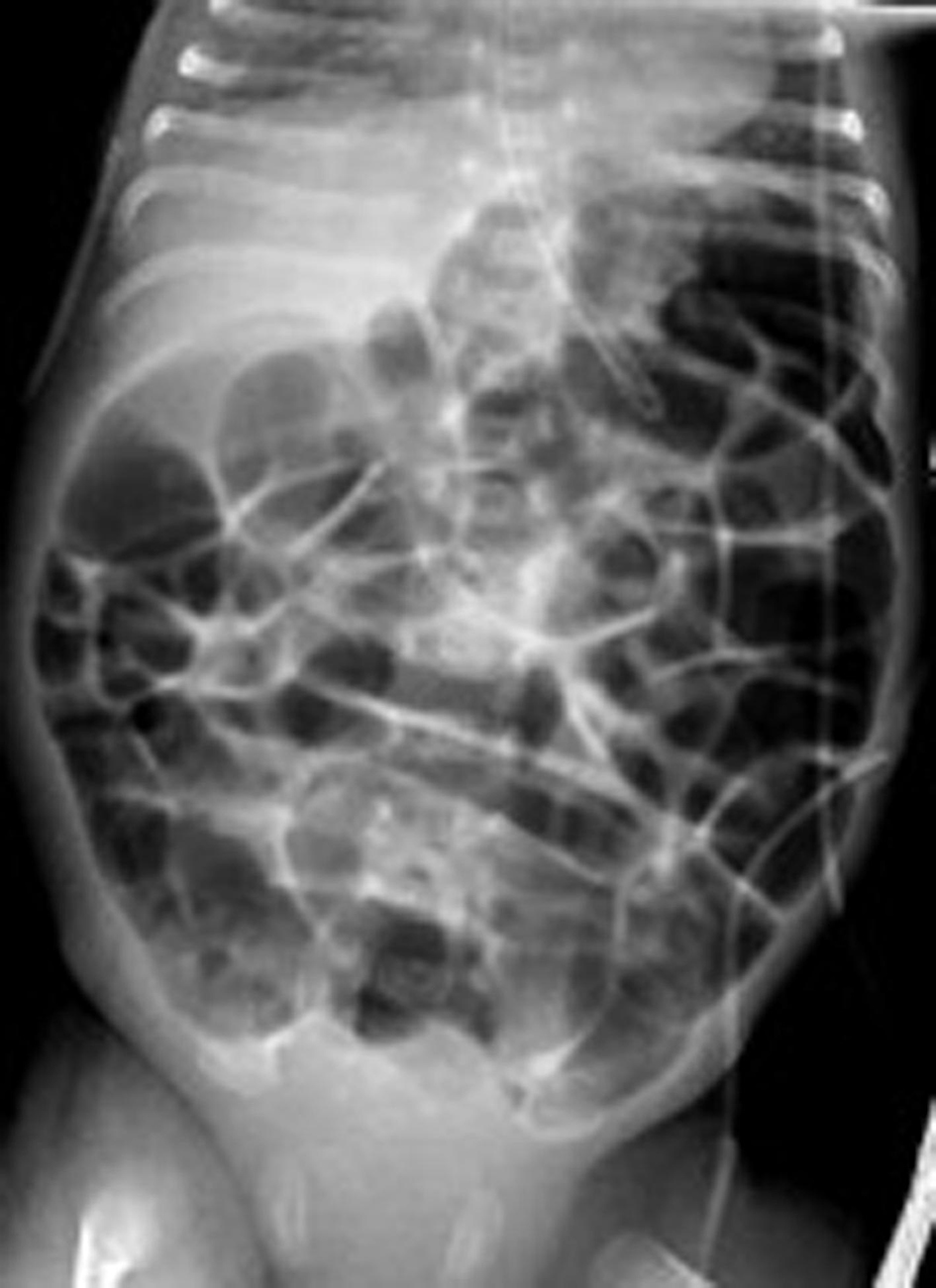 Click for large image | Figure 1. Chest and abdominal X-ray at 3 weeks of age, respiratory support with bubble CPAP of 8 cm of H2O and 6 L/min of gas flow in 25-30% FiO2 showing a normal distribution of the bowel gas pattern. CPAP: continuous positive airway pressure. |
On day 37 (33 weeks corrected), AJ developed firm, massively distended abdomen, which had worsened. Over the next 12 h, he developed feed intolerance with significant non-bilious gastric aspirates without vomiting. He was receiving 170 mL/kg/day of 24 kcal/30 mL expressed breast milk along with human milk fortifier. He developed episodic desaturations, until a “crisis-point” occurred when he suddenly became profoundly cyanosed with oxygen saturations of 50-60% needing 100% FiO2. This desaturation episode was unresponsive to intermittent positive pressure ventilation due to massively distended abdomen causing no chest wall rise and required immediate intubation. The abdominal circumference had increased by 3 cm over 12-h period compared to previous measurements 3 weeks ago. A capillary blood gas showed significant respiratory acidosis (pH 7.12, PCO2 80 mm Hg), which was a change to that recorded 24 h prior (pH of 7.32, PCO2 of 52 mm Hg). Due to high clinical suspicion of NEC, AJ underwent urgent chest and abdominal X-ray with blood tests to rule out sepsis. The chest X-ray was unremarkable and abdominal X-ray did not show evidence of pneumatosis intestinalis or portal venous gas. The bowel gas pattern was well distributed throughout, with thin-wall, dilated loops containing excessive air. There were no signs of intestinal perforation on the X-ray (Fig. 2a, b).
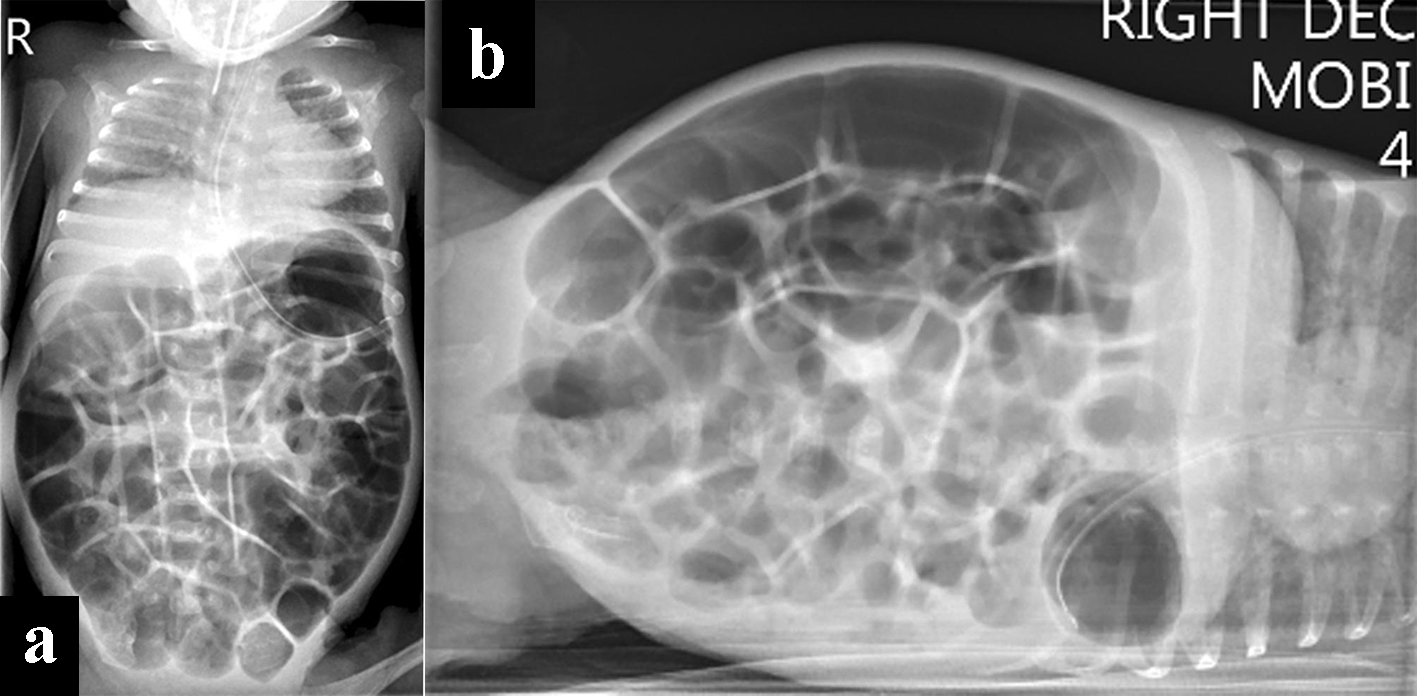 Click for large image | Figure 2. Chest and abdominal X-rays. (a) At 5 weeks of age, was on respiratory support with bubble CPAP of 7 cm of H2O and 6 L/min of gas flow in 25-30 % FiO2 with the diagnosis of CPAP belly syndrome, immediately post-intubation. (b) Decubitus right body side up lateral shoot through X-ray film with no evidence of perforation. CPAP: continuous positive airway pressure. |
There was no history of bloody or watery stools with no concerns of oral or nasal secretions and no vomiting during or before the episode. Chest X-ray showed no signs of aspiration and the intubating operator did not report any milk visible in the oropharynx. There was no history suggestive of gastro-esophageal reflux. A gastric tube with continuous free drainage was placed and feeding was withheld. AJ received empiric antibiotics with intravenous vancomycin (15 mg/kg/dose, 12 hourly for 5 days) and gentamicin (5 mg/kg/dose once a day for 5 days). His Hb was 104 g/L with a reticulocyte count of 5%. AJ attained cardio-pulmonary stability within 2 - 3 h of intubation and the capillary gas improved remarkably by 6 h (PCO2 55 mm Hg). His lactate levels remained normal (< 2 mmol/L) throughout this episode. There was no rise in inflammatory markers (C-reactive protein and pro-calcitonin), and his liver function, renal function and thyroid functions tests were normal. His blood cultures remained sterile during this episode.
Invasive ventilation, cessation of enteral feeds, continuous venting of gastric air using open-air drainage of the oro-gastric tube resulted in decompression of the abdominal distension. AJ’s abdominal girth returned to its baseline measurement within 36 h. Plain abdominal X-ray showed improved bowel gas pattern with less distended bowel loops (Fig. 3). After 5 days of invasive ventilation, AJ was extubated back to bubble CPAP with commencement of enteral feeding. He subsequently demonstrated good weight gain.
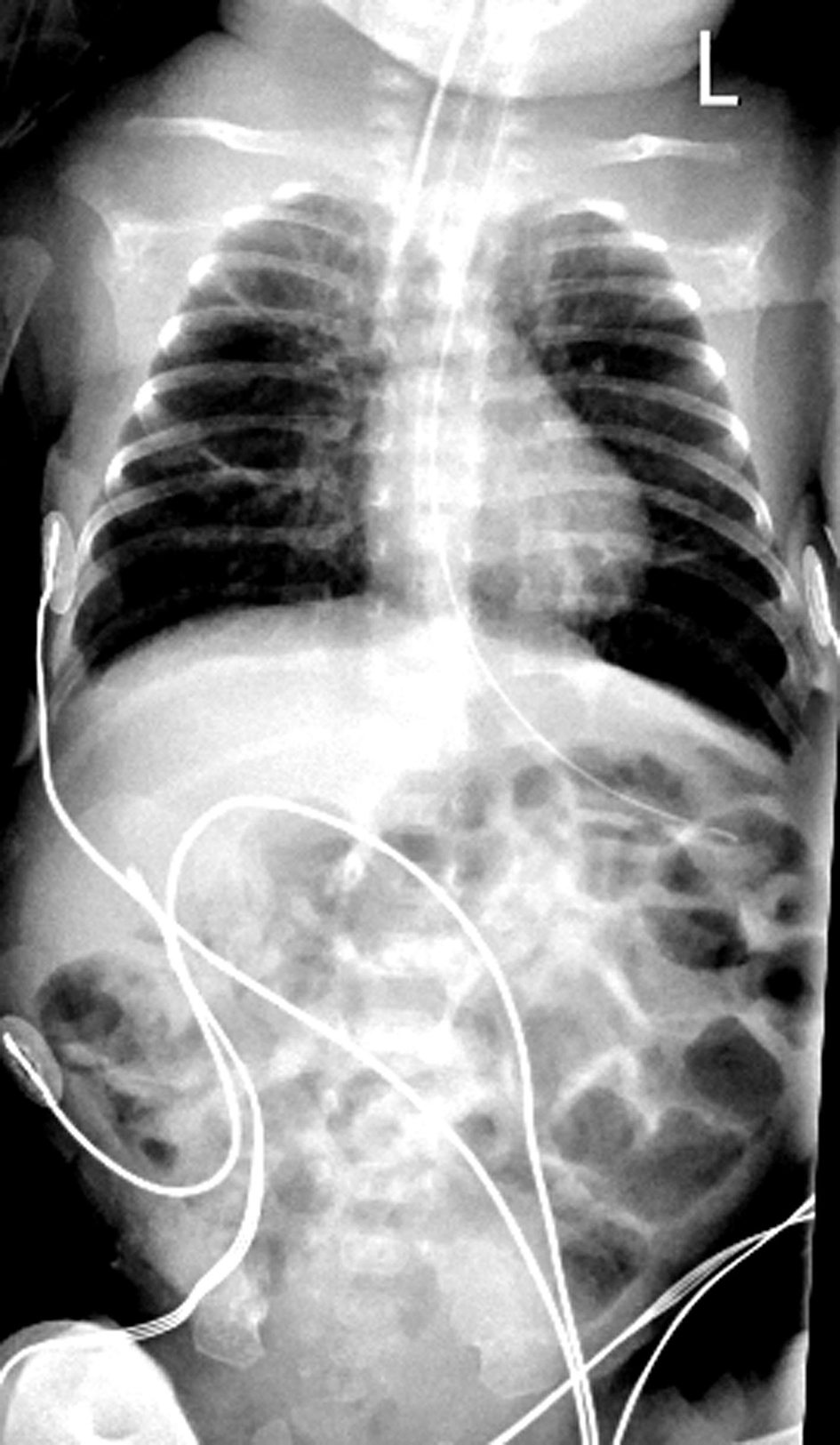 Click for large image | Figure 3. Chest and abdominal X-ray at 36 h post-intubation for CPAP belly syndrome showing reduced bowel gas pattern (decompression post-intubation, cessation of feeds) in comparison to Figure 2. CPAP: continuous positive airway pressure. |
In retrospect, the explanation for AJ’s acute clinical deterioration was attributed to severe CPAP belly syndrome. His abdominal examination and X-rays improved remarkably after abdominal decompression measures.
His subsequent stay in the neonatal intensive care unit remained uncomplicated. He received CPAP of 7 cm of H2O and 6 L/min of flow in 25% FiO2 until 42 weeks gestation, followed by supplemental ultra-low flow oxygen for another 2 weeks. AJ was diagnosed with CLD, given his ongoing oxygen requirement and chest X-ray findings at 36 weeks corrected gestational age. He was discharged home without the need for any respiratory support or home oxygen supplementation at 43 weeks gestation.
AJ was followed up in our Growth and Development Clinic due to his prematurity. He demonstrated his growth and developmental milestones within the expected range for his corrected gestational age with no concerns.
| Discussion | ▴Top |
The clinical presentation of CPAP belly syndrome in our case was contrary to the previously known benign description of this condition. While intubating and ventilating to decompress the abdomen was a clinical priority, there was a pressing need to screen for more serious intestinal conditions such as NEC.
There is a growing trend in neonatal intensive care units to avoid intubation and ventilation in extreme preterm infants by optimizing non-invasive respiratory support with CPAP [2]. To achieve this lung-protective strategy, the delivery of CPAP must be effective. The mouth forms a natural leak for nasal CPAP systems; hence, chin straps are often deployed. Inherently nose seal and mouth leaks are variable, resulting in unavoidable transmission of variable pressure and flow of gas into the pharyngeal area, esophagus, and ultimately into the gastrointestinal tract lumen. Higher pressures and flows, particularly with bi-level positive airway pressure (BiPAP), is even more likely to deliver gas into the gastrointestinal tract lumen.
CPAP belly syndrome is episodic and a multifactorial event (Fig. 4). CPAP belly syndrome occurs in active infants and thus is not seen in the immediate postnatal period due to relative periods of inactivity observed in extreme preterm infants [7]. Extreme preterm infants have an immature gastrointestinal tract with delayed gastric emptying time. Gastric emptying time is significantly reduced in very low birth weight (VLBW) infants treated with CPAP when compared to infants not receiving any respiratory support [8]. This is possibly due to the lung distension associated with CPAP exerting pressure on the diaphragm, which then exerts further downward pressure on gastric contents, reducing gastric volume and affecting its function (gastric emptying time) (Fig. 5) [7]. Excessive swallowed air distends the stomach and subsequently the intestines, creating increased intra-abdominal pressure with the need to further increase CPAP to overcome the abdominal pressure effects, resulting in a vicious cycle.
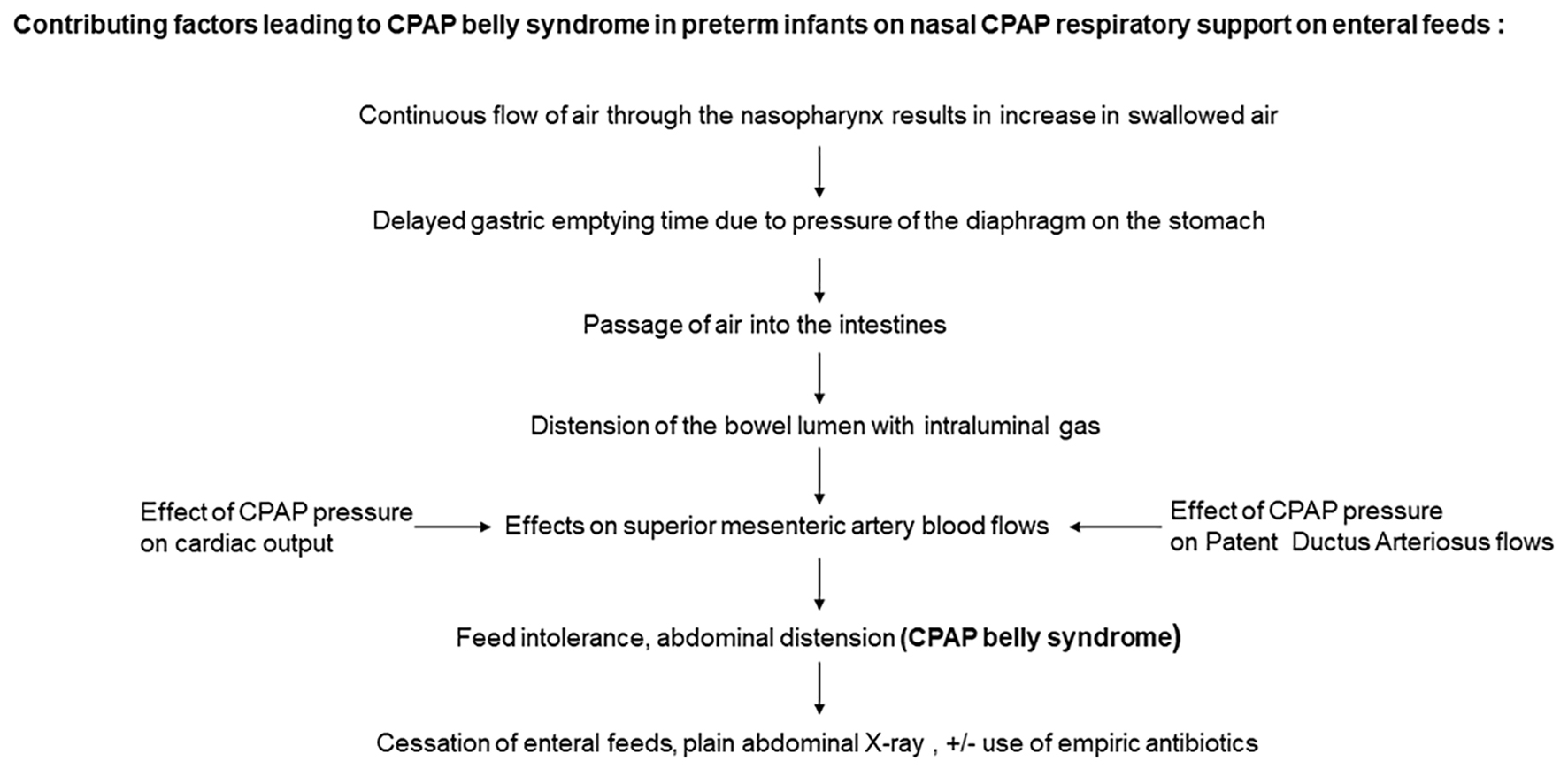 Click for large image | Figure 4. Flow chart on the pathogenesis of CPAP belly syndrome. CPAP: continuous positive airway pressure. |
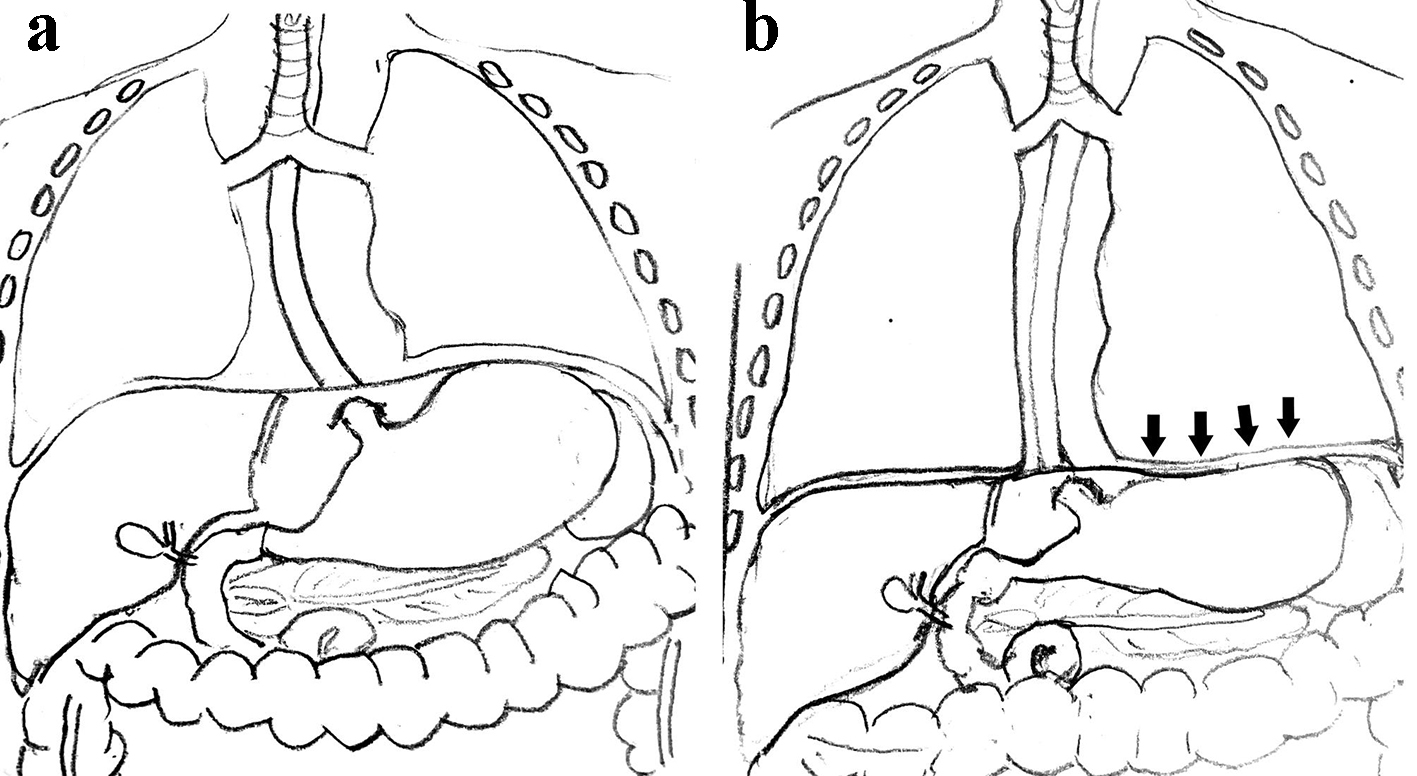 Click for large image | Figure 5. (a) Normal diaphragm and gastric contents. (b) Arrows indicate the downward pressure effects of CPAP on the diaphragm exerting pressure on the gastric contents, reducing gastric emptying time. CPAP: continuous positive airway pressure. |
In addition to intra-abdominal pressure effects from gaseous bowel distension, continuous use of CPAP affects intestinal blood flow adversely [9]. The blood flow parameter of the superior mesenteric artery (SMA) is an early predictor of intestinal dysmotility in preterm infants [10]. Typically, there is an increase in the pre-prandial SMA blood flow velocity between days 1 and 5 postnatal age in VLBW infants. However, with the use of CPAP, this increase in the SMA blood flow velocity is attenuated, resulting in intestinal dysmotility and feed intolerance [11, 12]. The data on SMA blood flow velocities are from VLBW infants on CPAP in their early postnatal period; however, characteristically in CPAP belly syndrome, distension does not occur in the first few days after birth. Further studies are required to assess SMA flow velocities during episodes of CPAP belly syndrome. Furthermore, the effects of PDA and cardiac output during episodes of CPAP belly syndrome require further research.
A few prophylactic measures tried to reduce gaseous bowel distension include frequent syringe suctioning of air from the gastric tube, use of an additional venting gastric tube and per-rectal use of glycerine suppositories. Despite these measures, the occurrence of CPAP belly syndrome is a common clinical scenario for neonatologists and there are no published guidelines on optimal feeding practices for extreme preterm infants on CPAP.
Severe CPAP belly syndrome presenting with marked abdominal distension, feed intolerance and desaturation episodes, often results in a compelling reason to exclude more severe pathological causes such as NEC, volvulus or intestinal perforation. Ceasing feeds and performing abdominal X-rays to exclude pneumatosis intestinalis are all justified. Increased intestinal dysmotility with early bacteremia or sepsis with Staphylococcus epidermidis or Gram-negative sepsis is also a clinical possibility. Thus, empirical antibiotics until sepsis is ruled out forms part of the clinical management bundle. Following a period of bowel rest and gastric deflation feeds are usually recommenced once pathological intestinal conditions are ruled out. Thus, CPAP belly syndrome may necessitate more clinical vigilance and assessments until an alternative diagnosis such as NEC is excluded.
Our case reflects the diagnostic challenge faced by neonatologists in managing extreme preterm infants on CPAP support and presenting with severe CPAP belly syndrome. The clinical interpretation of the case and investigations led to the diagnosis of severe CPAP belly syndrome, as a diagnosis of exclusion. We acknowledge, however, that we did not perform any viral studies on secretions or stools to exclude the less common diagnosis of viral colitis as an alternative explanation. Also, the usual range of CPAP pressures is reported as 4 - 6 cm H2O, and AJ was initially managed on 7 cm H2O, and increased to 8 cm H2O. Thoracoabdominal asynchrony from hyperinflation, due to higher CPAP, is a clinical possibility. There was no evidence of hyperinflation on the chest X-ray during the episode of CPAP belly syndrome. On the other hand, the chest expansion was suboptimal at 4½ intercostal spaces, due to gross abdominal distension. Whether merely using higher CPAP of 8 cm of H2O predisposed AJ to develop severe CPAP belly syndrome is still plausible. Further research on the association of higher CPAP pressures and CPAP belly syndrome is required.
Diagnosis of CPAP belly syndrome
Differentiating CPAP belly syndrome from early NEC remains a diagnostic challenge. Clinical improvement in abdominal distension over time guides management, which often occurs following gastrointestinal rest once feeds are withheld. Plain abdominal radiography is the gold standard investigation to ascertain normal bowel gas pattern. However, this comes at the cost of significant cumulative irradiation [13]. Further studies are required to determine whether other methods of assessment of bowel health can be used to confirm CPAP belly. These methods include assessing cerebro-splanchnic oxygenation index using near-infrared spectroscopy (NIRS) with long-term bowel sound analysis methods in time series. Unlike these proposed research areas, point-of-care abdominal US (Fig. 6) is an excellent screening tool.
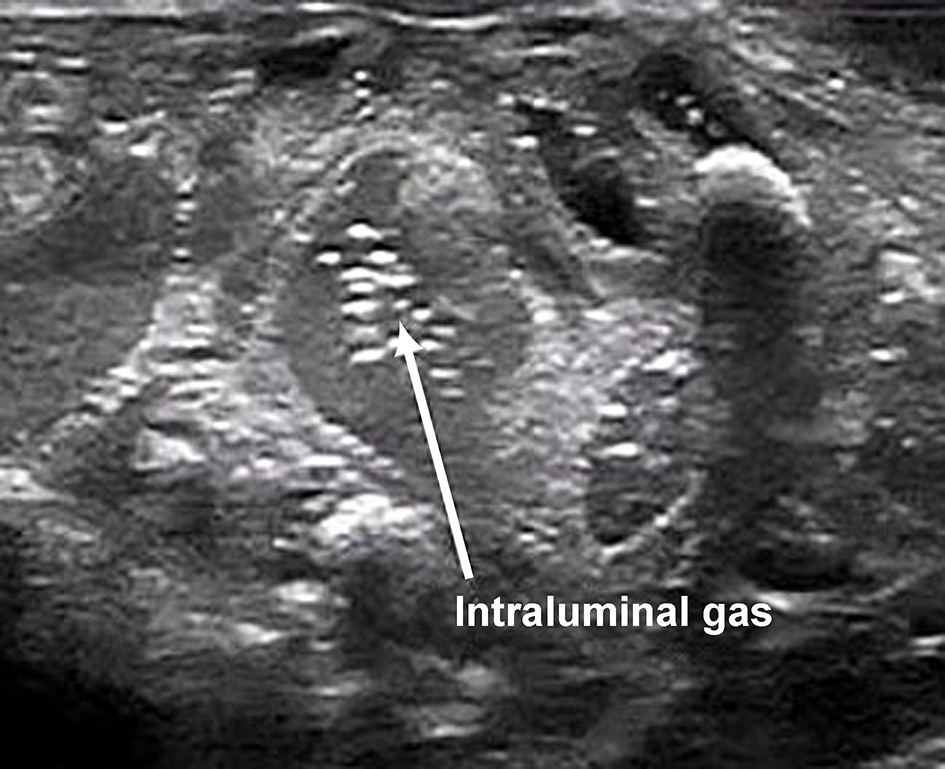 Click for large image | Figure 6. Normal bowel wall ultrasound finding. |
Abdominal US assessments of bowel wall thickness or thinness, vascularity, peristalsis and intraabdominal fluid collections can add valuable diagnostic information to differentiate CPAP belly from NEC. Abdominal US during episodes of severe CPAP belly syndrome may be challenging because of intrusive gas artifacts. Despite the gas reverberation artifacts obscuring bowel images, peristalsis is often easy to appreciate. Application of gentle pressure on the transducer or placing the transducer in the flanks along with slight lateral tilting of the patient may be helpful in visualization of the bowel loops. Detection of standard “gut signature”, peristalsis and normal vascularity are a sign of a healthy bowel and reassuring to the clinician. Point-of-care abdominal US in the diagnosis of NEC has shown a definitive utility in the hands of pediatric radiologists [14, 15]. Our group is exploring the utility of neonatologist performed point-of-care abdominal US to exclude NEC from CPAP belly syndrome, and determine the real negative and positive predictive value of its utility. Further research is needed to redefine the description of CPAP belly syndrome, prevalence estimation and more potent measures to prevent and treat its occurrence in extreme preterm infants.
Conclusions
Despite its challenges, CPAP is an effective form of respiratory support in the management of preterm infants. CPAP belly syndrome in preterm infants is currently an inevitable sequel to the use of CPAP. Measures in prevention of accumulation of gas in the stomach and facilitating passage of regular stools are vital in its prevention. This case demonstrates the potential for clinical deterioration leading to escalation in respiratory support to invasive ventilation, use of empiric antibiotics and gastrointestinal rest. CPAP belly syndrome in its severe form is not a benign condition. This condition warrants vigilant monitoring and differentiation from progressive severe gastrointestinal conditions demanding escalation of treatment which is justified until conditions such as NEC, volvulus and perforation are ruled out. With improvements in respiratory care of extreme preterm infants, we face the challenges of the changing paradigm of CPAP belly syndrome. Further studies are required to objectively define CPAP belly syndrome and evaluate the role of point-of-care abdominal US assessment of the bowel in reassuring clinicians of the absence of severely diseased bowel states.
Acknowledgments
We thank the parents for providing us with the encouragement and permission to publish this clinical case. We would like to thank Dr Traci-Anne Goyen and Dr Marc Kelly, Department of Neonatology, Westmead Hospital, for their contribution in editing the manuscript.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was obtained from the parent (father) of the patient for publication of this case report.
Author Contributions
AP wrote up manuscript, literature review, obtained patient consent, prepared the figures and coordinated manuscript reviewing by all authors. MH critically analyzed the manuscript, formatted and edited manuscript figures as per submission guidelines. NB critically analyzed the manuscript. ML critically analyzed the manuscript. MT designed the concept, supervised construction of the literature review, critically analyzed the manuscript, formal supervisor to AP. All authors read and approved the final manuscript.
Abbreviations
BiPAP: bi-level positive airway pressure; CPAP: continuous positive airway pressure; RDS: respiratory distress syndrome; CLD: chronic lung disease; FiO2: fraction of inspired oxygen; NEC: necrotizing enterocolitis; VLBW: very low birth weight; SMA: superior mesenteric artery; NIRS: near-infrared spectroscopy; US: ultrasound
| References | ▴Top |
- Birenbaum HJ, Dentry A, Cirelli J, Helou S, Pane MA, Starr K, Melick CF, et al. Reduction in the incidence of chronic lung disease in very low birth weight infants: results of a quality improvement process in a tertiary level neonatal intensive care unit. Pediatrics. 2009;123(1):44-50.
doi pubmed - Clark RH, Gerstmann DR, Jobe AH, Moffitt ST, Slutsky AS, Yoder BA. Lung injury in neonates: causes, strategies for prevention, and long-term consequences. J Pediatr. 2001;139(4):478-486.
doi pubmed - Van Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, Susser M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics. 2000;105(6):1194-1201.
doi pubmed - Davis PG, Morley CJ, Owen LS. Non-invasive respiratory support of preterm neonates with respiratory distress: continuous positive airway pressure and nasal intermittent positive pressure ventilation. Semin Fetal Neonatal Med. 2009;14(1):14-20.
doi pubmed - Lee KS, Dunn MS, Fenwick M, Shennan AT. A comparison of underwater bubble continuous positive airway pressure with ventilator-derived continuous positive airway pressure in premature neonates ready for extubation. Biol Neonate. 1998;73(2):69-75.
doi pubmed - Tagare A, Kadam S, Vaidya U, Pandit A, Patole S. Bubble CPAP versus ventilator CPAP in preterm neonates with early onset respiratory distress—a randomized controlled trial. J Trop Pediatr. 2013;59(2):113-119.
doi pubmed - Jaile JC, Levin T, Wung JT, Abramson SJ, Ruzal-Shapiro C, Berdon WE. Benign gaseous distension of the bowel in premature infants treated with nasal continuous airway pressure: a study of contributing factors. AJR Am J Roentgenol. 1992;158(1):125-127.
doi pubmed - Gounaris A, Costalos C, Varchalama L, Kokori P, Kolovou E, Alexiou N. Gastric emptying in very-low-birth-weight infants treated with nasal continuous positive airway pressure. J Pediatr. 2004;145(4):508-510.
doi pubmed - Havranek T, Thompson Z, Carver JD. Factors that influence mesenteric artery blood flow velocity in newborn preterm infants. J Perinatol. 2006;26(8):493-497.
doi pubmed - Robel-Tillig E, Knupfer M, Pulzer F, Vogtmann C. Blood flow parameters of the superior mesenteric artery as an early predictor of intestinal dysmotility in preterm infants. Pediatr Radiol. 2004;34(12):958-962.
doi pubmed - Maruyama K, Koizumi T, Tomomasa T, Morikawa A. Intestinal blood-flow velocity in uncomplicated preterm infants during the early neonatal period. Pediatr Radiol. 1999;29(6):472-477.
doi pubmed - Fang S, Kempley ST, Gamsu HR. Prediction of early tolerance to enteral feeding in preterm infants by measurement of superior mesenteric artery blood flow velocity. Arch Dis Child Fetal Neonatal Ed. 2001;85(1):F42-45.
doi pubmed - Olgar T, Onal E, Bor D, Okumus N, Atalay Y, Turkyilmaz C, Ergenekon E, et al. Radiation exposure to premature infants in a neonatal intensive care unit in Turkey. Korean J Radiol. 2008;9(5):416-419.
doi pubmed - Priyadarshi A, Rogerson S, Hinder M, Tracy M. Neonatologist performed point-of-care bowel ultrasound: Is the time, right? Australasian Journal of Ultrasound in Medicine. 2019;22(1):15-25.
doi - Epelman M, Daneman A, Navarro OM, Morag I, Moore AM, Kim JH, Faingold R, et al. Necrotizing enterocolitis: review of state-of-the-art imaging findings with pathologic correlation. Radiographics. 2007;27(2):285-305.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
