| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Case Report
Volume 9, Number 1, March 2020, pages 4-8
Lemierre’s Syndrome: A Case Report of a Child With Partial Treatment of Streptococcal Pharyngitis
Joshua Pryora, Priya Sharmab, Rachel A. Reedyc, d
aUniversity of Florida College of Medicine, Gainesville, FL, USA
bDepartment of Radiology, University of Florida Health, Gainesville, FL, USA
cDepartment of Pediatrics, University of Florida Health, Gainesville, FL, USA
dCorresponding Author: Rachel Reedy, Department of Pediatrics, University of Florida Health, 7046 SW Archer Rd, Gainesville, FL 32608, USA
Manuscript submitted September 23, 2019, accepted October 25, 2019
Short title: Lemierre’s Syndrome
doi: https://doi.org/10.14740/ijcp347
| Abstract | ▴Top |
Lemierre’s syndrome (LS) is a complication of acute oropharyngeal infections leading to septic thrombophlebitis of the internal jugular vein. Since initial discovery, the incidence of LS has decreased due to development of modern antibiotics. In our case report, a child with partially treated streptococcal pharyngitis presented with neck pain, neck swelling and limited range of motion of her neck. Although the patient was well appearing and with an atypical presentation of LS, the diagnosis of LS was confirmed with a computed tomography (CT). As a result, appropriate treatment was quickly initiated to prevent further complication and provide a favorable outcome for the patient. Typical presenting symptoms of LS include fever, dysphagia, neck pain and arthralgia that should lead to the ordering of appropriate laboratory studies and imaging. CT imaging aids in both the diagnosis and management of LS. The use of anticoagulation therapy remains controversial in the management of LS. In addition, there are no generalized guidelines for the treatment of LS, but antibiotic therapy directed at anaerobic coverage can significantly reduce morbidity and mortality in otherwise healthy children. Due to potentially fatal complications, it is crucial to keep LS on the differential diagnosis list in pediatric patients with fever, neck pain, neck swelling, or dysphagia in the week following an oropharyngeal infection.
Keywords: Lemierre’s syndrome; Oropharyngeal infection; Thrombophlebitis; Internal jugular vein
| Introduction | ▴Top |
Lemierre’s syndrome (LS) is a potentially fatal complication of acute oropharyngeal infections leading to septic thrombophlebitis of the internal jugular vein [1, 2]. This disease often affects individuals between the age of 16 and 30 years with an estimated incidence of 0.8 per million people [1, 3].
At its time of discovery in 1936 by Andre-Alfred Lemierre, LS was often fatal due to complications of sepsis [2, 4]. With the discovery of penicillin in 1928, and more importantly its commercial use in the treatment of oropharyngeal infections in the mid-to-late 1940s, the incidence of LS dropped significantly [2, 5]. Between 1950 and 1990, cases were sparsely reported; however, in the 1990s, the number of cases reported increased [3, 5]. It is postulated that this increased reporting was due to restrictive prescribing patterns for acute pharyngitis/tonsillitis and acute otitis media, as well as the development of improved blood culture techniques to better capture anaerobic bacteria [3, 5]. Due to the development of modern antibiotics, LS is now appropriately known as “the forgotten disease” [1, 3, 5].
We herewith present a case of LS in a pediatric patient with an atypical presentation. This patient presented with a complaint of neck pain and neck swelling. However, after further history taking, abnormal examination findings and laboratory results, the diagnosis of LS was made with a confirmatory computed tomography (CT). This led to appropriate treatment and a favorable outcome for the patient with prevention of further complications.
| Case Report | ▴Top |
A 16-year-old female patient, with no significant past medical history, presented to her primary care physician with a 2-week history of right-sided neck swelling that had worsened over the past 2 days. Associated, constant, right-sided neck pain acutely started 2 days ago when she woke up; the pain radiated up to the right ear and down to the right shoulder. The pain was worse with all neck movements. She had taken 400 mg of ibuprofen as needed with temporary improvement of the neck pain. Additional symptoms included an improving sore throat and an intermittent tactile fever for 3 weeks.
She was seen 3 weeks prior in the emergency department (ED) for similar symptoms, at which time she was not taking any medications. In the ED, she was diagnosed with streptococcal pharyngitis, by a positive rapid point of care group A streptococcus test, and influenza, by a positive rapid point of care influenza test, unsure if diagnosis was influenza A or influenza B as results were from an outside ED. There was not a throat culture sent to the lab. At that time, she was prescribed a 5-day course of azithromycin and a 5-day course of oseltamivir 30 mg; however, she completed 500 mg of azithromycin on day 1 and 250 mg of azithromycin on day 2 then discontinued the azithromycin due to emesis after dosing. A week after the ED visit, due to her persistent sore throat, she was given one dose each of ketorolac, ceftriaxone and methylprednisolone by a family member employed in the medical field with the intention of treating her streptococcal pharyngitis; the doses of these medication are unknown. The sore throat resolved for 4 days before returning with the accompaniment of right-sided neck pain, which instigated her to return to the ED. During her second ED visit, she had a negative monospot test and was given a single injection of 1,200,000 units of penicillin G to treat her suspected partially treated streptococcal pharyngitis; there was no point of care test or throat culture performed. She was instructed to follow up with her primary care physician if symptoms persisted; these brought her into clinic at this time.
There was no pertinent family, social, or other past medical history. She was up to date on immunizations, except for the influenza vaccine. Her last vaccines were Tdap and influenza vaccines administered 4 years prior. She did not smoke or consume alcohol. There was no recent travel history, known allergies, or known exposures. Otherwise, a review of systems was negative. On physical exam, in her primary care clinic, her vitals were: weight 96.5 kg, temperature 36.9 °C, blood pressure 126/78 mm Hg, heart rate 120 beats per minute and oxygen saturation 99% on room air. She was well appearing and in no appreciable distress. The right side of her neck was significantly tender to palpation, edematous without fluctuance, warm and non-erythematous. Neck pain and limited range of motion of the neck was noted with neck flexion, extension, left rotation and left lateral bend. There were no palpable lymph nodes and no meningeal signs. On oral examination, she had moist mucous membranes and tonsils without swelling, exudates, or erythema. The remaining physical exam was within normal limits.
Given her history and acute worsening of symptoms in the past 2 days, she was sent back to the ED for imaging to rule out an underlying thrombophlebitis or abscess. In the ED, a CT of neck (Fig. 1a-c) was performed and showed pharyngitis with right internal jugular vein thrombophlebitis with complete occlusion, which was consistent with LS. There was no drainage, fluid collection, or abscess formation noted on the CT.
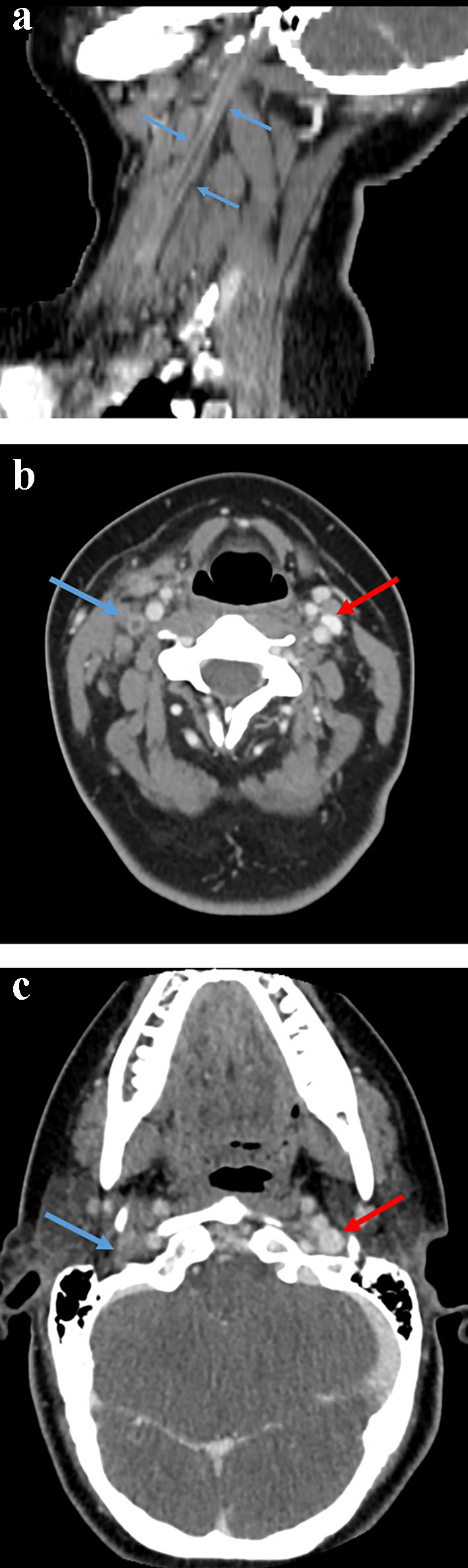 Click for large image | Figure 1. (a) Contrast-enhanced computed tomography image of the neck in the sagittal plane demonstrating the thrombosed right internal jugular vein (blue arrows). (b) Contrast-enhanced computed tomography axial image of neck showing the thrombosed right internal jugular vein (blue arrow) compared to the patent left internal jugular vein (red arrow). (c) Axial contrast-enhanced computed tomography at the base of the skull at the level of the jugular foramen. The right internal jugular vein is thrombosed (blue arrow) compared to the patent left internal jugular vein (red arrow). |
After her CT was resulted, she was admitted to the hospital for further evaluation and management. At the time of admission, her vital signs were: temperature 36.9 °C, blood pressure 97/63 mm Hg, heart rate 91 beats per minute and oxygen saturation 100% on room air. Her physical exam had not changed from earlier that day in her primary care clinic. There were no rapid tests, cultures, or repeat mononucleosis test performed at time of admission. Laboratory results showed an elevated C-reactive protein, elevated erythrocyte sedimentation rate, thrombocytosis, normal liver enzymes (Table 1) and a normal urinalysis. Laboratory results did not show any atypical eosinophils on the complete blood count (CBC). Otolaryngology was consulted and recommended against surgical intervention due to the lack of abscess formation. Per recommendation by an infectious disease consult, she was treated with 3 days of intramuscular ceftriaxone, 2 g every 24 h, and intravenous clindamycin, 15 mg/kg every 8 h for 14 days, followed by oral clindamycin 450 mg three times a day for an additional 28 days. The ceftriaxone was discontinued after three doses, once blood cultures from admission were negative. Hematology consult recommended against anti-coagulation therapy. After a 14-day hospital stay and symptom improvement, she was discharged home with infectious disease follow-up. She completed 6 weeks of oral clindamycin during which time she was followed closely by infectious disease; however, with resolution of her symptoms after 6 weeks of antibiotic therapy, she was instructed to continue follow up only as needed. She was also followed monthly by hematology/oncology for 6 months; during those 6 months, she had no interim ED visits for neck pain or swelling and her D-dimer remained within normal limits suggesting no clot activity. Three years later, she has had no complications or symptoms due to her previous diagnosis of LS and has not needed to follow up with any pediatric specialty department.
 Click to view | Table 1. Patient’s Laboratory Results With Associated Reference Ranges at the Time of Diagnosis of Lemierre’s Syndrome |
| Discussion | ▴Top |
This patient was a 16-year-old female who presented with right-sided neck pain, neck swelling, limited range of motion secondary to pain and subjective fever after being partially treated for streptococcus pharyngitis. A CT then confirmed the diagnosis of LS. Nearly all patients with fever and imaging confirmed LS have signs of septic emboli, namely cough, pleuritic chest pain, tachypnea, or hemoptysis, all of which were absent in this patient [5]. As a result, definitive imaging searching for septic emboli was not done due to lack of clinical suspicion.
The most common implemented etiologic agent of LS is Fusobacterium spp., in particular Fusobacterium necrophorum, though Streptococcus spp. and Staphylococcus aureus have also been reported [2]. One study of 96 patients with LS confirmed by imaging and pathogen growth with blood culture showed that 52% of cases were caused by Fusobacterium spp., 18% Streptococcus spp., 6.3% Staphylococcus aureus, with the remaining 23.7% due to other, often gram-negative, bacteria [2].
Fusobacterium spp. are pleomorphic gram-negative curved rods that are commensal flora of the alimentary canal, especially the oral cavity and pharynx [1-3, 5]. Fusobacterium spp. are non-motile, filamentous, obligate anaerobes [1, 3]. These bacteria are considered opportunistic pathogens because they do not invade intact mucosal membranes [1, 3]. However, when a host’s mucosal surfaces are damaged through oropharyngeal infections by other pathogens, Fusobacterium spp. can invade the parapharyngeal space [1, 3, 5, 6]. Once in the parapharyngeal space, this organism uses several virulence factors including lipopolysaccharide, hemagglutinin, hemolysin, lipase and leukocidin to facilitate further infiltration into the IJV through the tonsillar vein or into local lymphatic channels causing thrombophlebitis [5, 7].
Presenting symptoms of LS, such as high fevers and rigors, commonly initiate 1 week following the onset of an oropharyngeal infection [2, 5, 7]. Neck pain, dysphagia and swelling at the angle of the mandible and parallel to the anterior border of the sternocleidomastoid will often accompany initial symptoms [1, 5, 6]. By the time fever has started, 79-100% of patients will already have septic emboli to the lungs resulting in cough, pleuritic chest pain, dyspnea/tachypnea and hemoptysis [2, 3, 5, 7]. Arthralgias are present in 11-27% of patients [1], liver enzyme elevation is found in approximately 50% of patients [1] and jaundice is reported in 11-49% of patients [6, 7]. Coagulopathies occur in 4% of patients but are usually mild and not clinically significant [1, 5]. Differential diagnosis of LS should include infective mononucleosis, parapharyngeal abscess, pneumonia, lymphoma, leukemia and diseases consistent with septic emboli such as endocarditis [1, 5, 6].
The diagnosis of LS is based on index of suspicion and should be considered with substantial neck swelling, respiratory involvement, or if early signs of toxicity/sepsis are present in the week succeeding an acute oropharyngeal infection [4-7]. In addition, a recent Epstein-Barr virus infection may induce immunosuppression with a transient decrease in T-cell-mediated immunity and may be a predisposing risk factor for LS or a more severe course of illness [8]. Laboratory studies may show increased acute phase reactants such as C-reactive protein or erythrocyte sedimentation rate [1-3, 5, 6], absolute neutrocytosis in the presence of leukocytosis, elevated liver enzymes and thrombocytopenia [1, 7]. CT imaging aids in both diagnosis and management of disease progression [3, 5-7]. The presence of thrombi in the IJV and cavitary lesions in the lung fields on contrast CT of the neck and chest are highly suggestive of LS [2, 5-7]. Fusobacterium spp. have been historically difficult to culture but their presence on blood or fluid culture confirm the diagnosis [2, 5, 6, 9]. Blood cultures should be drawn prior to the initiation of empiric antibiotic therapy [2, 5, 6].
Antibiotics should be directed at anaerobic coverage [5-7]. Fusobacterium spp. have 100% sensitivity to metronidazole, imipenem, cefoxitin and ticarcillin-clavulanate [10], and are resistant to quinolones and gentamicin [5-7]. Monotherapy with metronidazole is not recommended due to the severity of the infection [5]. Clindamycin has been effective in treatment of septic emboli and lung abscesses, though susceptibilities should be examined first because of resistance patterns [1, 5, 6]. Most causative agents are shown to have beta-lactamase activity, therefore beta-lactamase resistant beta-lactams are recommended for empiric treatment [2, 5, 6]. In recent years, beta-lactamase resistant beta-lactams have been recommended for empiric treatment due to treatment failures and increasing beta-lactam resistance [5, 6]. There are no generalized guidelines for the treatment of LS, leading to variability in treatment [5-7]. Patients are generally treated for 2 weeks with intravenous antibiotic (or longer depending on severity of illness) and then transitioned to oral antibiotics for a total of 4 - 6 weeks of therapy [2, 3, 5-7]. Antibiotics should be narrowed as soon as susceptibilities are received [1, 5, 6].
The use of anticoagulation therapy remains controversial in LS management [1-3, 6, 7, 9]. There have been no randomized controlled clinical trials testing the efficacy of anticoagulation therapy and thus there are no universally adopted guidelines [1, 6, 7, 9]. Most pediatric patients in recent years have received some form of anticoagulation therapy as an attempt to prevent or slow thrombus expansion into the cavernous sinus [1, 5, 7, 9, 11]. Some studies state that anticoagulation therapy could reduce production of septic emboli, though the evidence is not overwhelming [3, 6, 9]. Therefore, treatment should focus on initiating appropriate antibiotic therapy as this is the only treatment proven to significantly decrease mortality to less than 10% [2, 5, 6, 9]. Cardiovascular and respiratory support may be required in severe cases of sepsis [4, 7]. Surgical excision of thrombus is rarely indicated [1, 5-7]. Peritonsillar or parapharyngeal abscesses, empyema of the lung and septic joints should be drained surgically after the initiation of antibiotic therapy [5-7].
Patients with untreated LS will progress to symptoms of metastatic infection with septic emboli that can seed bones, meninges, lung parenchyma, blood vessels and heart valves [1, 2, 5-7]. Untreated LS can lead to serious neurologic (meningitis), cardiac (endocarditis), respiratory (septic pulmonary emboli), skeletal complications (osteomyelitis and septic joint) and even death for an otherwise healthy child [1, 5, 6]. If treated the mortality of LS is 2% [2] but delayed treatment of 4 or more days has been shown to have fatality between 10% and 25% [1, 2, 6, 7] and meningeal infection has been shown to have a fatality of approximately 30% [7].
Conclusion
LS is a potentially serious complication occurring in the weeks following oropharyngeal infections. This case highlights an atypical presentation of LS that was confirmed with CT imaging which leads to rapid treatment and a full recovery of the patient. Early antibiotic therapy directed at anaerobic coverage with or without the use of anticoagulation can significantly reduce morbidity and mortality in otherwise healthy children.
Acknowledgments
None to declare.
Financial Disclosure
Joshua Pryor, Priya Sharma and Rachel Reedy have disclosed no financial relationship relevant to this article and no specific funding.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Joshua Pryor drafted the initial manuscript, formatted the table, reviewed and revised figures and final draft of the manuscript. Priya Sharma reviewed and edited figures and critically reviewed and revised the manuscript. Rachel Reedy participated in direct care of the patient in clinic and critically reviewed and revised the manuscript.
| References | ▴Top |
- Riordan T. Human infection with Fusobacterium necrophorum (Necrobacillosis), with a focus on Lemierre's syndrome. Clin Microbiol Rev. 2007;20(4):622-659.
doi pubmed - Johannesen KM, Bodtger U. Lemierre's syndrome: current perspectives on diagnosis and management. Infect Drug Resist. 2016;9:221-227.
doi pubmed - Hagelskjaer Kristensen L, Prag J. Human necrobacillosis, with emphasis on Lemierre's syndrome. Clin Infect Dis. 2000;31(2):524-532.
doi pubmed - Tawa A, Larmet R, Malledant Y, Seguin P. Severe sepsis associated with Lemierre's syndrome: a rare but life-threatening disease. Case Reports Crit Care. 2016;2016:1-3.
doi pubmed - Rae J, Misselbrook K. Lemierre's syndrome - a rare cause of disseminated sepsis requiring multi-organ support. J Intensive Care Soc. 2017;18(4):329-333.
doi pubmed - Allen BW, Bentley TP. Lemierre Syndrome. In: StatPearls. Treasure Island (FL), 2019.
- Lu MD, Vasavada Z, Tanner C. Lemierre syndrome following oropharyngeal infection: a case series. J Am Board Fam Med. 2009;22(1):79-83.
doi pubmed - Chacko EM, Krilov LR, Patten W, Lee PJ. Lemierre's and Lemierre's-like syndromes in association with infectious mononucleosis. J Laryngol Otol. 2010;124(12):1257-1262.
doi pubmed - McGouran D, Keene A, Walklin R, Carter J. A complex case of bilateral Lemierre syndrome with suggestions on anticoagulation management. Intern Med J. 2013;43(6):728-730.
doi pubmed - Appelbaum PC, Spangler SK, Jacobs MR. Beta-lactamase production and susceptibilities to amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, cefoxitin, imipenem, and metronidazole of 320 non-Bacteroides fragilis Bacteroides isolates and 129 fusobacteria from 28 U.S. centers. Antimicrob Agents Chemother. 1990;34(8):1546-1550.
doi pubmed - Repanos C, Chadha NK, Griffiths MV. Sigmoid sinus thrombosis secondary to Lemierre's syndrome. Ear Nose Throat J. 2006;85(2):98-101.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
