| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Original Article
Volume 8, Number 1, August 2019, pages 1-7
Nutritional Management of Gastroesophageal Reflux Among Infants in the Philippines: Insights From Real-World Evidence
Felizardo Gatchecoa, Maria Imelda Vitug Salesb, Grace Battadc, Marilou Tand, Ma Cecilia D. Gloriae, Urszula Kudlaf, Leilani Muhardig, h
aJose R. Reyes Memorial Medical Center, Manila, Philippines
bMakati Medical Center, Manila, Philippines
cUniversity of the East Ramon Magsaysay Medical Center, Manila, Philippines
dPhilippine Children Medical Center, Manila, Philippines
eMarketing Department, FrieslandCampina, Manila, Philippines
fFrieslandCampina, Amersfoort, the Netherlands
gMedical Affairs Department, FrieslandCampina AMEA Pte Ltd, Singapore
hCorresponding Author: Leilani Muhardi, FrieslandCampina AMEA Pte Ltd, 3 Temasek Avenue, #11-01 Centennial Tower, 039190, Singapore
Manuscript submitted July 15, 2019, accepted August 9, 2019
Short title: Nutritional Management of GER in Infants
doi: https://doi.org/10.14740/ijcp338
| Abstract | ▴Top |
Background: The objective of the study was to describe the clinical course of infants with pediatrician-diagnosed gastroesophageal regurgitation (GER), after changing to an infant anti-regurgitation formula.
Methods: Information on frequency and volume of regurgitation, and disease progression were collected from mothers of 0- to 12-month-old Filipino infants with GER at baseline and 1 month after a pediatrician prescribed-formula containing carob bean gum, galacto-oligosaccharides and partially hydrolyzed whey protein for 14 days.
Results: Eighty-nine infants aged ≤ 6 months and 40 aged 7 - 12 months old were enrolled. The most frequently reported amount of baseline regurgitation was half of the total feed (29 (33%) younger infants and 17 (43%) older infants). Baseline regurgitation frequency ranged from 1 - 3 times/day (45 (51%) and 21 (52%)) to 4 - 6 times/day (33 (37%) and 14 (35%)) and 7 - 9 times/day (11 (12%) and five (13%)). Regurgitation after 1-day consumption was resolved in 16 (18%) and 8 (20%) and in 57 (64%) and 31 (78%) younger and older infants at 14 days. Forty-one (32%) infants still had regurgitation episodes after a 14-day trial with decreased frequency and volume; three (7%) infants did not show any improvement, while one (1%) infant had increased amount of regurgitation. No medicine was given to study participants. Parent-reported sleep disturbance decreased in three (37%) younger infants and 25 (63%) older infants.
Conclusion: Nutrition intervention has effectively improved symptom and quality of life among infants with GER within 14 days. Information on underlying conditions among those with unresolved symptoms are needed.
Keywords: Anti-regurgitation; Carob bean gum; Galacto-oligosaccharides; Gastroesophageal regurgitation; Infants; Partially hydrolyzed whey protein; Special infant milk formula
| Introduction | ▴Top |
Gastroesophageal reflux (GER) is one of the most common digestive problems in the first months of life and decreases the quality of life in formula-fed infants [1]. The worldwide prevalence of infantile GER is 30% [1] with a peak of 67-87% at 2 - 4 months of life [2]. A survey conducted in China, Malaysia, Russia and Vietnam showed that this disorder affected 61% infants aged between 0 and 3 months, 32% infants aged 3 - 6 months and 8% infants aged 6 - 12 months [3].
Infantile GER is usually diagnosed using the new symptom-based Rome IV criteria for infants and toddlers [2]. In parallel, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) have developed an international consensus to help the pediatricians and pediatric subspecialists in the diagnosis and management of infantile GER [4]. A joint consensus between NASPGHAN and ESPGHAN jointly recommends non-pharmacological intervention with lifestyle changes in the infants such as thickened feedings, consumption of special anti-regurgitation formulas and positioning after meals [4].
Data support the use of thickened formula with added functional ingredients like carob bean gum (CBG), galacto-oligosaccharides (GOS) and partially hydrolyzed whey proteins (PHW) [5-12]. CBG is an indigestible but fermentable fiber with prebiotic effects [10, 13], in which its mechanism of action involves increasing the viscosity of food and milk at a safe therapeutic level of 0.5 g/100 mL [6], resulting in a reduction in total and acid reflux [10, 13]. Moreover, CBG may promote the development of more diverse microbiota and a lower pH as compared to rice or maize starch [10]. GOS have been reported to have bifidogenic effects, promote the maturity of the gastrointestinal tract and improve the stool consistency at 0.24 g/L, 0.4 g/L and 0.5 g/L [7-9, 14]. In parallel, PHW has been associated with fast gastric emptying time and improve digestion in infants [11].
No real-world data are available, for example, on the duration to symptom resolution in infants suffering from GER. In this context, a survey was conducted to understand the clinical course of Filipino infants (0 - 12 months) who were suffering from infantile GER diagnosed by a pediatrician and received an anti-regurgitation infant milk formula containing CBG, GOS and PHW.
| Patients and Methods | ▴Top |
Design and data collection
An observational and questionnaire-based survey was conducted between November 2017 and June 2018 in the Greater Manila Area, North Luzon and South Luzon in the Philippines. Fifty pediatricians were asked to recommend a 14-day trial of a special anti-regurgitation infant milk formula (Frisolac® AR, FrieslandCampina, the Netherlands) containing CBG, GOS and PHW to infants aged between 0 and 12 months and diagnosed with GER (Table 1). Regurgitation in this study was defined as any occurrence of refluxing gastric contents after feeds more than three times a day but no associated historical or physical examination findings were abnormal. Exclusion criteria were exclusively breastfed infants, normal healthy infants without any signs and symptoms of regurgitation and infants with obvious underlying structural deformities related to regurgitation.
 Click to view | Table 1. Nutritional Profile of the Special Anti-Regurgitation Infant Milk Formula |
Institutional Review Board approval was not obtained as this is a survey with products already in the market as part of the nutrition management. Ethical compliance with human/animal study was not obtained. Verbal consent was obtained from the survey’s participants prior to their participation but not recorded. The survey instrument, a questionnaire, was completed orally with the parents and filled by the pediatricians at baseline, 14 days and 30 days after a special nutrition intervention to evaluate the improvement of the symptoms using the same tool. No information could be obtained unless the parents agreed to participate, and no data could be filled by pediatricians unless released by the parents themselves. The questionnaire collected frequency and volume of regurgitation, progression of the disease and other complaints including the reported volume of regurgitation (Supplementary 1, www.theijcp.org).
The product used for the nutritional management of GER has received regulatory approval and is available in the Philippines since 2016 and in other markets since 2010.
Statistical analyses
Descriptive analyses were performed using counts and frequencies (n, %) and using IBM SPSS version 24 for Windows release (SPSS, Statistical Packages for Social Sciences, an IBM Statistical Software, IBM Corp., Armonk, NY).
| Results | ▴Top |
Patients demographic
A total of 135 infants diagnosed with GER were prescribed the special infant formula during the survey period. Six patients were excluded from the analysis because four patients did not meet the selection criteria (aged 1 - 3 years), and pediatricians did not provide the date of birth or the age of two patients. Therefore, data of 129 eligible infants (0 - 12 months old) were analyzed: 89 (69%) infants aged 0 - 6 months and 40 (31%) infants aged 7 - 12 months.
Baseline characteristics
At baseline, 45 (51%) younger infants aged 0 - 6 months and 21 (52%) older infants aged 7 - 12 months regurgitated 1 - 3 times a day. Thirty-three (37%) younger infants and 14 (35%) older infants regurgitated 4 - 6 times a day. A regurgitation frequency of 7 - 9 times a day was reported in 11 (12%) younger infants and five (13%) older infants.
The most frequently reported amount of regurgitation was half of the total feed in 29 (33%) younger infants and 17 (43%) older infants, followed by a mouthful in 26 (29%) younger infants and 12 (30%) older infants. All of the feed was regurgitated by 13 (15%) younger infants and two (5%) older infants (Fig. 1).
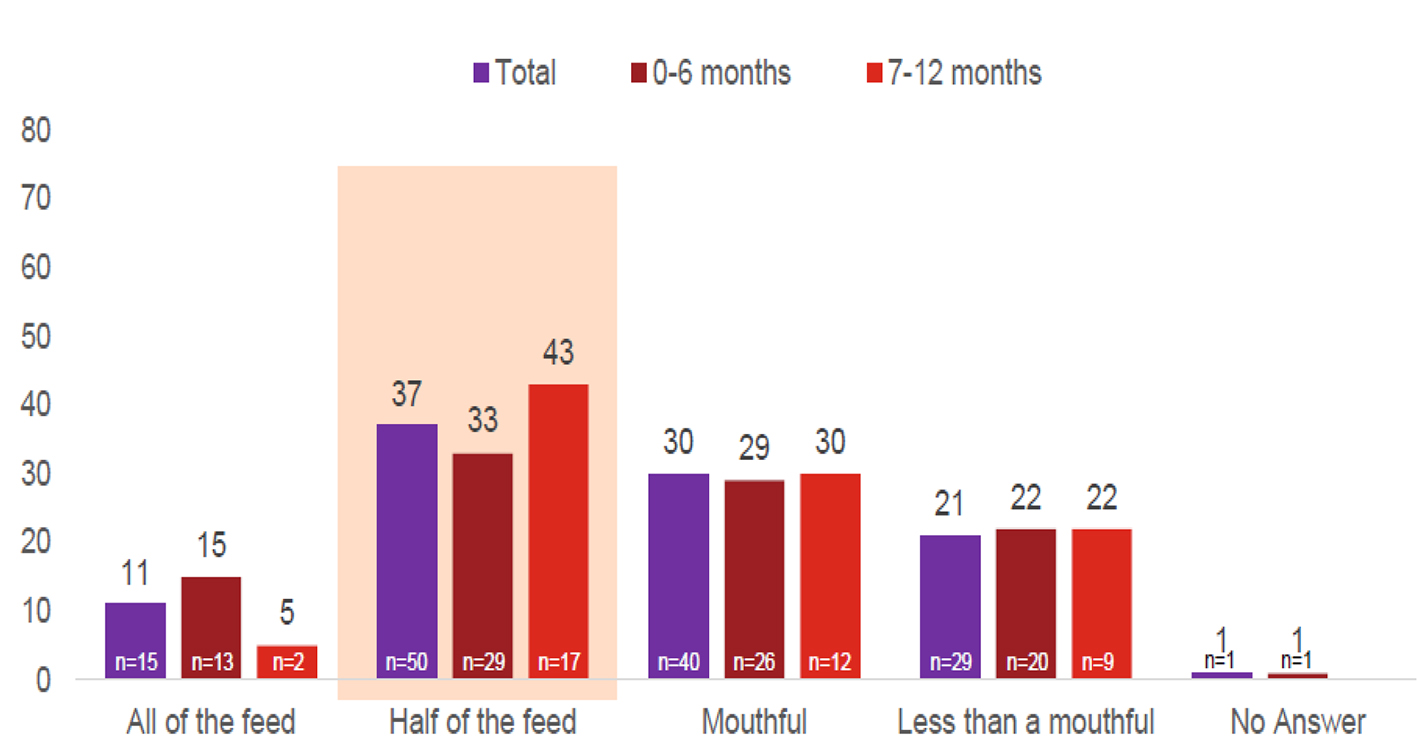 Click for large image | Figure 1. Reported average of regurgitation volume at baseline (%). Total interviews (n = 135); 0 - 6 months (n = 89); 7 - 12 months (n = 40). |
Endline characteristics after using the special infant formula
After 1 day of special formula consumption, 16 (18%) younger infants and eight (20%) older infants had no more regurgitation. No regurgitation at the frequency of 7 - 9 episodes of regurgitation was reported and the number of younger infants regurgitating 4 - 6 times daily decreased to eight (9%). More infants (n = 65; 73%) were regurgitating 1 - 3 times daily; a similar pattern was observed for the older age group (7 - 12 months) (Fig. 2a). Likewise, the reported number of infants regurgitating half of the total feed decreased to 12 (13%) in the 0- to 6-month age group and to one (3%) in the 7- to -12-month age group. More infants (n = 52 (58%) in younger and n = 25 (62%) in older age groups) reported regurgitating less than a mouthful (Fig. 2b).
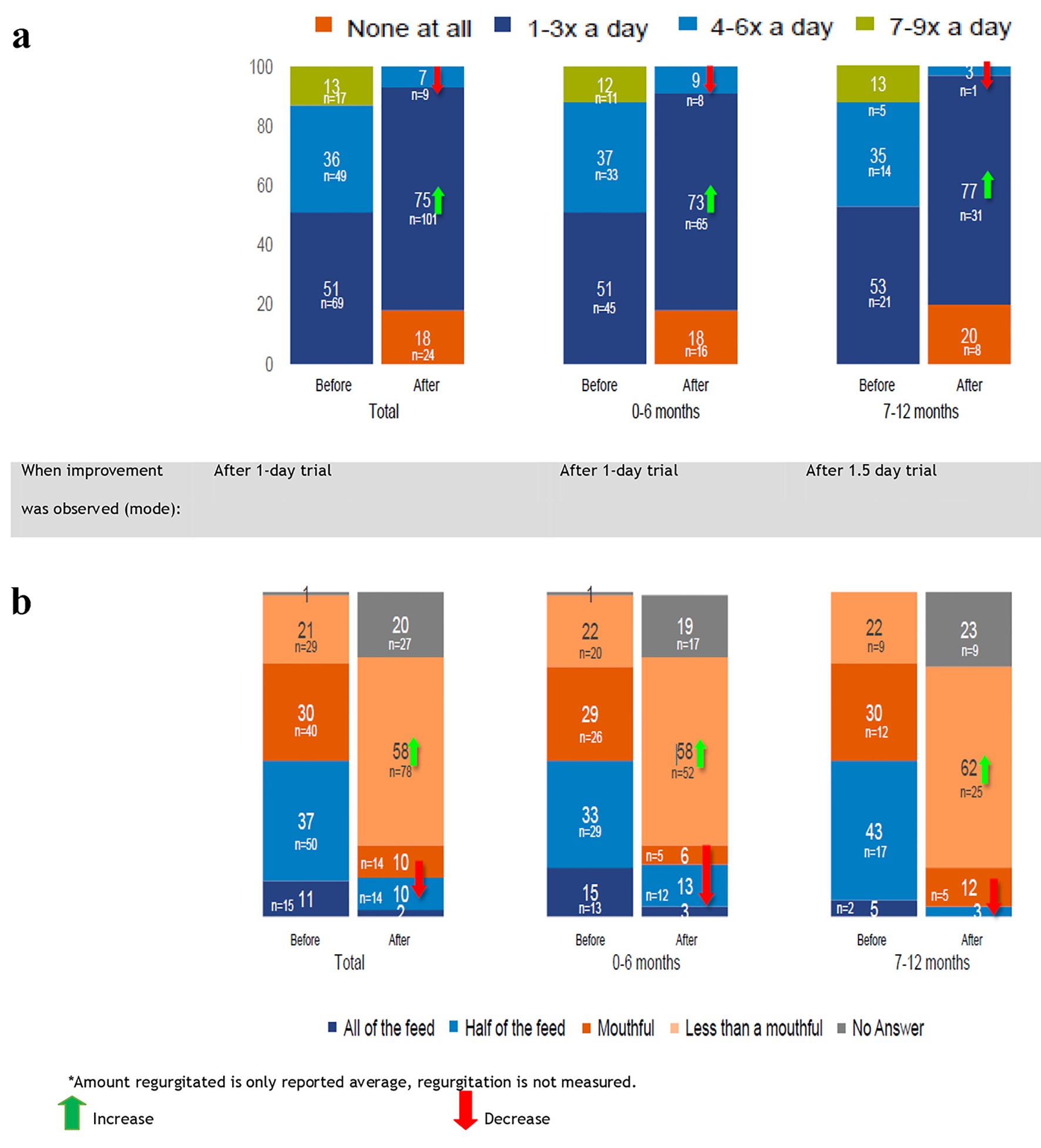 Click for large image | Figure 2. (a) Average frequency of regurgitation after using the special infant formula (%). (b) Average amount usually regurgitated after using the special infant formula (%). Total interviews (n = 135); 0 - 6 months (n = 89); 7 - 12 months (n = 40). |
Between day 1 and day 5 of special formula consumption, decreased frequency and amount of regurgitation was more evident. More infants (n = 29 (73%) in younger and n = 47 (53%) in older age groups) were reported to have a decreased volume of regurgitation. After 14 days, regurgitation was completely resolved among the majority of the patients: 57 (64%) younger infants and 31 (78%) older infants. However, 41 (32%) infants continued regurgitation episodes after a 14-day trial but the frequency and volume of regurgitation decreased in 26 (64%) younger infants and 24 (59%) older infants. Three (7%) infants did not show any improvement, and one (1%) infant had an increased amount of regurgitated feed (Fig. 3).
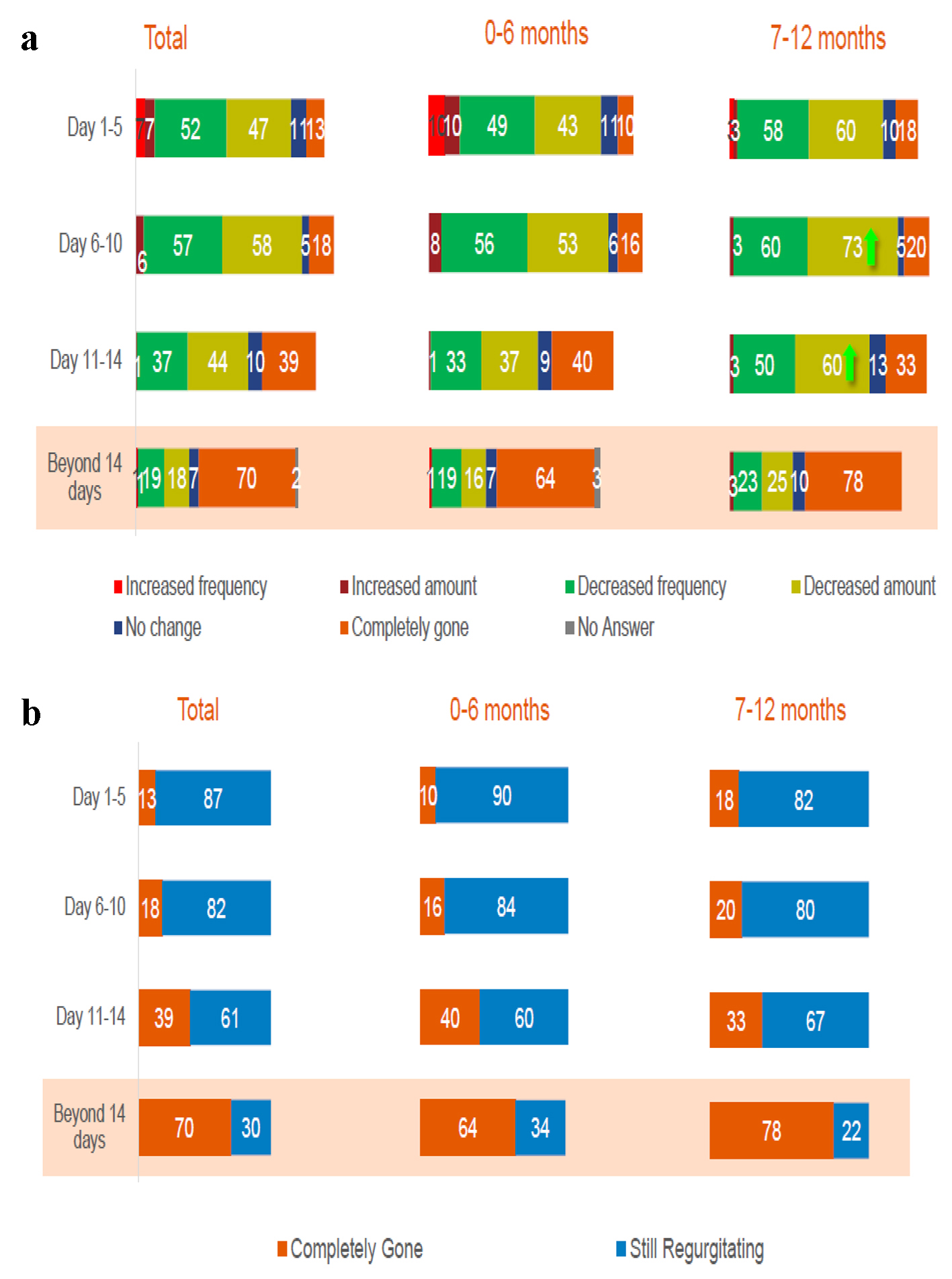 Click for large image | Figure 3. (a, b) Symptoms’ improvement at 14 days of consumption (%). Total interviews (n = 135); 0 - 6 months (n = 89); 7 - 12 months (n = 40). |
On the quality of life’s questionnaire, parent reported decreased sleep disturbance for 33 (37%) younger infants and 25 (63%) older infants and decreased incessant crying for 45 (51%) younger infants and 26 (65%) older infants. Additionally, bowel movement was improved in 48 (54%) younger infants and 24 (60%) older infants, and longer feeding time was reported among 27 (30%) younger infants and 13 (33%) older infants (Fig. 4).
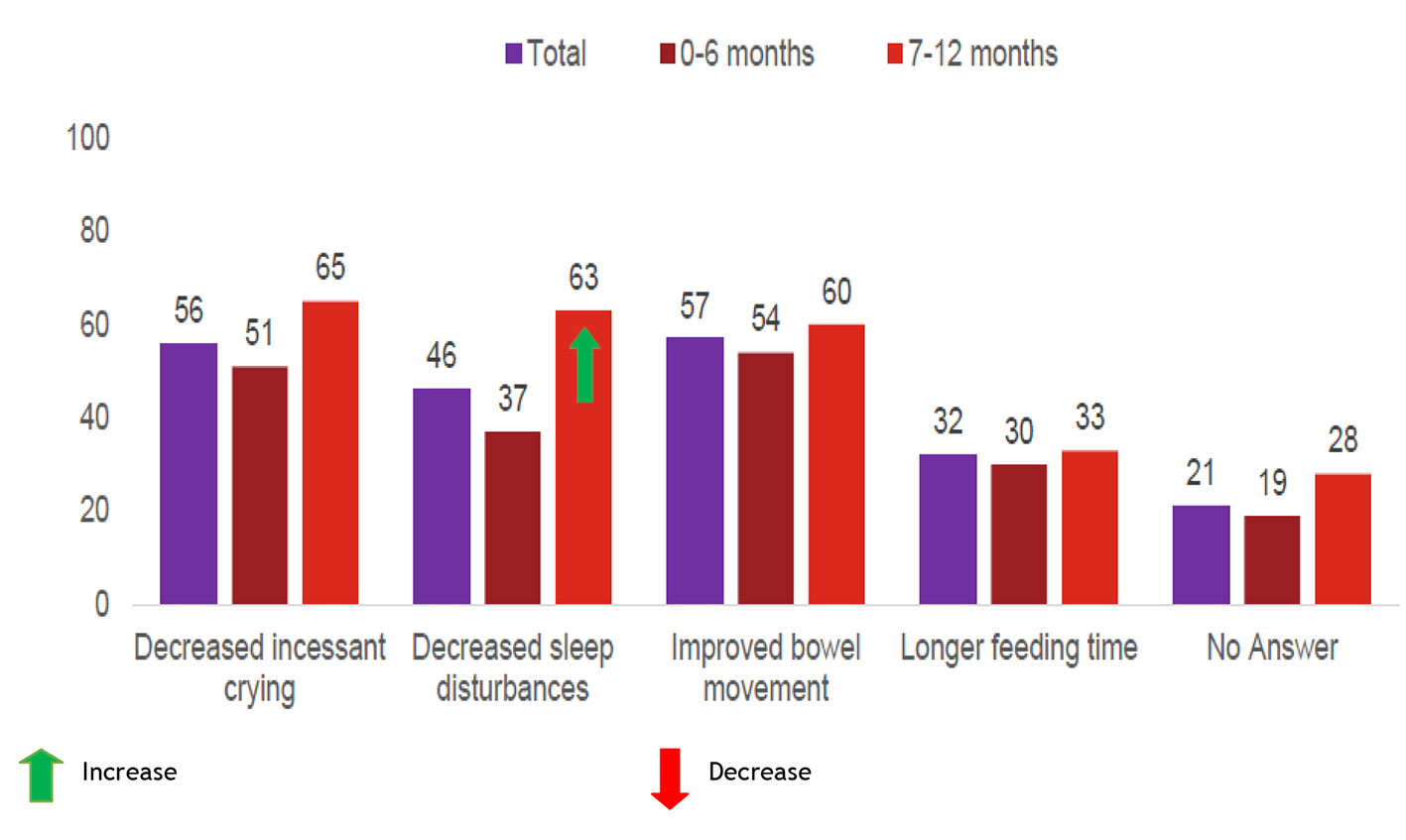 Click for large image | Figure 4. Other effects in 14-day trial (%). Total interviews (n = 135); 0 - 6 months (n = 89); 7 - 12 months (n = 40). |
| Discussion | ▴Top |
This survey revealed that GER-related symptoms such as regurgitation still occur among infants aged 7 - 12 months (31% of all infants) [15]. Generally, GER peaks at the age of 2 - 4 months [2, 15] and should resolve by 12 months of age.
Nutritional intervention seems effective in the management of GER without any medicine as one out of five infants no longer had regurgitation within 1 day of shifting to the anti-regurgitation formula; outcomes tended to be similar in younger and older infants. Furthermore, the amount of regurgitation decreased to less than a mouthful across all ages as claimed by the patients’ mothers. Further symptom’s resolution was reported between 1 and 5 days of usage as half of the infants showed an improvement in the frequency and amount of regurgitation. Such effects are likely to have a positive influence on parental anxiety and quality of life especially among first-time parents [16, 17].
The percentage of the study participants without any regurgitation increased from 13% at days 1 - 5, to 18% at days 6 - 10, to 39% at days 11 - 14 and to an average of 70% (64% of younger infants and 78% of older infants) at 14 days of using this formula (Fig. 3a).
The consumption of the anti-regurgitation infant milk formula containing CBG, GOS and PHW was also associated with an improvement in the quality of life of the infants as incessant crying and sleep disturbances. Regular and softer stools were also reported particularly among the infants aged 7 - 12 months consuming the formula as this could be effective in the management of multiple symptoms of functional gastrointestinal disorders in early life [1].
The results of this observational survey are consistent with the findings of previous clinical studies on anti-regurgitation infant milk formulas containing the same thickening agents [18, 19]. A randomized and partly double-blind clinical trial compared the same infant milk formula at 0.33 g/100 mL of cold CBG (formula A, Frisolac® AR) with two other formulas at 0.45 g/100 mL of cold or hot CBG (formulas B and C). Formula A was shown to be more effective in decreasing the occurrence of reflux episodes and improving clinical symptoms of GER (decreased night cough, crying and frequency of vomiting) after 2 weeks of intervention [18]. Also in a prospective, double-blind, randomized cross-over trial conducted in 115 formula-fed infants (2 weeks to 5 months old), a formula containing CBG at 0.33 g/L and hydrolyzed protein was more effective in reducing the number and volume of regurgitations compared to another formula containing a nonhydrolyzed protein and less CBG (0.33 g/L) [11].
This survey has some limitations. First, it was not a randomized trial, thus it did now allow any direct comparison with a control group. Second, only children from three regions of the Philippines were included; however, our population can be seen as representative of an urban population. Third, this survey might be affected by the self-reporting bias as parents tend to over-report behaviors and symptoms viewed as appropriate by the researchers or doctors, and to under-report what is perceived to be inappropriate. Indeed, self-reporting bias is common in dietary surveys of infant feeding practices, especially those conducted verbally [20]. However, the same questionnaire was used at various time points in the survey to evaluate the improvement of the symptoms using the same tool. Fourth, data on the amount of formula given at each feeding, feeding methods (i.e. allowing the infants to burp and rest during the feeding) and their uniformity, and whether the infants were receiving other food items during the feeding with the formula including breast milk are not available. Regardless of these limitations, this survey described real-world insights on the time for symptom’s resolution and the effectiveness of nutrition management of GER among infants in the Philippines without any pharmacological intervention. It also provided a window of opportunity for non-medical based intervention in functional regurgitation leading to improved parents’ quality of life.
To conclude, this survey investigated the clinical course of Filipino infants suffering from infantile GER, after changing to a special infant formula designed to relieve GER. A formula designed for management of regurgitation and thickened with CBG, GOS and PHW effectively improved regurgitation symptoms and patient’s quality of life within the 14 days of consumption. Underlying conditions among infants who still had symptoms after 14 days need to be investigated, especially that prolonged symptoms of functional gastrointestinal disorders in early life could lead to increased risk of these disorders in later life [21].
Acknowledgments
The authors are thankful for the infants and their families who participated in this survey. The authors also thank Content Ed Net Singapore for providing editorial and medical writing assistance for the preparation of this manuscript. They also thank GfK Philippines Cooperation for data collection and analysis.
Financial Disclosure
The authors, Felizardo Gatcheko, Maria Imelda Vitug Sales, Grace Battad and Marilou Tan received an educational grant from Friesland Campina to review and publish the survey’s result.
Conflict of Interest
Ma Cecilia D. Gloria, Urszula Kudla and Leilani Muhardi are employees of Friesland Campina.
Informed Consent
Verbal consent was obtained from the survey’s participants prior to the survey.
Author Contributions
Ma Cecilia D. Gloria designed and monitored the study implementation. Felizardo Gatcheko, Maria Imelda Vitug Sales, Grace Battad, Marilou Tan, Urszula Kudla and Leilani Muhardi reviewed the study results and interpretation. All authors provided inputs and agreed on the final version of the manuscript.
| References | ▴Top |
- Bellaiche M, Oozeer R, Gerardi-Temporel G, Faure C, Vandenplas Y. Multiple functional gastrointestinal disorders are frequent in formula-fed infants and decrease their quality of life. Acta Paediatr. 2018;107(7):1276-1282.
doi pubmed - Zeevenhooven J, Koppen IJ, Benninga MA. The New Rome IV criteria for functional gastrointestinal disorders in infants and toddlers. Pediatr Gastroenterol Hepatol Nutr. 2017;20(1):1-13.
doi pubmed - Friso Healthcare Professional Survey. Cimigo 2010. Friso survey conducted in China, Malaysia, Russia and Vietnam in 2010 amongst 541 healthcare professionals.
- Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, Gupta S, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66(3):516-554.
doi pubmed - Horvath A, Dziechciarz P, Szajewska H. The effect of thickened-feed interventions on gastroesophageal reflux in infants: systematic review and meta-analysis of randomized, controlled trials. Pediatrics. 2008;122(6):e1268-1277.
doi pubmed - Meunier L, Garthoff JA, Schaafsma A, Krul L, Schrijver J, van Goudoever JB, Speijers G, et al. Locust bean gum safety in neonates and young infants: an integrated review of the toxicological database and clinical evidence. Regul Toxicol Pharmacol. 2014;70(1):155-169.
doi pubmed - Ben XM, Li J, Feng ZT, Shi SY, Lu YD, Chen R, Zhou XY. Low level of galacto-oligosaccharide in infant formula stimulates growth of intestinal Bifidobacteria and Lactobacilli. World J Gastroenterol. 2008;14(42):6564-6568.
doi pubmed - Giovannini M, Verduci E, Gregori D, Ballali S, Soldi S, Ghisleni D, Riva E, et al. Prebiotic effect of an infant formula supplemented with galacto-oligosaccharides: randomized multicenter trial. J Am Coll Nutr. 2014;33(5):385-393.
doi pubmed - Fanaro S, Marten B, Bagna R, Vigi V, Fabris C, Pena-Quintana L, Arguelles F, et al. Galacto-oligosaccharides are bifidogenic and safe at weaning: a double-blind randomized multicenter study. J Pediatr Gastroenterol Nutr. 2009;48(1):82-88.
doi pubmed - Gonzalez-Bermudez CA, Lopez-Nicolas R, Peso-Echarri P, Frontela-Saseta C, Martinez-Gracia C. Effects of different thickening agents on infant gut microbiota. Food Funct. 2018;9(3):1768-1778.
doi pubmed - Vandenplas Y, Leluyer B, Cazaubiel M, Housez B, Bocquet A. Double-blind comparative trial with 2 antiregurgitation formulae. J Pediatr Gastroenterol Nutr. 2013;57(3):389-393.
doi pubmed - Indrio F, Riezzo G, Giordano P, Ficarella M, Miolla MP, Martini S, Corvaglia L, et al. Effect of a partially hydrolysed whey infant formula supplemented with starch and lactobacillus reuteri DSM 17938 on regurgitation and gastric motility. Nutrients. 2017;9(11):1181.
doi pubmed - Herrewegh A. Effect van Frisovom flesvoeding op darmflora van zuigelingen. (Unpublished report in Dutch). NIZO report no. E2000/33; 2000.
- Stewart ML, Schroeder NM. Dietary treatments for childhood constipation: efficacy of dietary fiber and whole grains. Nutr Rev. 2013;71(2):98-109.
doi pubmed - Hegar B, Satari DH, Sjarif DR, Vandenplas Y. Regurgitation and gastroesophageal reflux disease in six to nine months old Indonesian infants. Pediatr Gastroenterol Hepatol Nutr. 2013;16(4):240-247.
doi pubmed - Salvatore S, Abkari A, Cai W, Catto-Smith A, Cruchet S, Gottrand F, et al. Review shows that parental reassurance and nutritional advice help to optimise the management of functional gastrointestinal disorders in infants [published online ahead of print, 2018 May 25]. Acta Paediatr. 2018;107(9):1512-1520.
doi
doi pubmed - Gonzalez Ayerbe JI, Hauser B, Salvatore S, Vandenplas Y. Diagnosis and management of gastroesophageal reflux disease in infants and children: from guidelines to clinical practice. Pediatr Gastroenterol Hepatol Nutr. 2019;22(2):107-121.
doi pubmed - Georgieva M, Manios Y, Rasheva N, Pancheva R, Dimitrova E, Schaafsma A. Effects of carob-bean gum thickened formulas on infants' reflux and tolerance indices. World J Clin Pediatr. 2016;5(1):118-127.
doi pubmed - Vivatvakin B, Buachum V. Effect of carob bean on gastric emptying time in Thai infants. Asia Pac J Clin Nutr. 2003;12(2):193-197.
- Donaldson S, Grant-Vallone E. Understanding self-report bias in organizational behavior research. J Bus Psychol. 2002;17:254-262.
doi - Bonilla S, Saps M. Early life events predispose the onset of childhood functional gastrointestinal disorders. Rev Gastroenterol Mex. 2013;78(2):82-91.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
