| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Original Article
Volume 6, Number 3-4, December 2017, pages 37-41
Local Cutaneous Complications After Bacille Calmette-Guerin Vaccine: Experience of a Single Center
Tugce Tural-Karaa, b, Halil Ozdemira, Tugba Erata, Aysun Yahsia, Ergin Ciftcia
aDepartment of Pediatric Infectious Diseases, Ankara University Medical School, Ankara, Turkey
bCorresponding Author: Tugce Tural-Kara, Department of Pediatric Infectious Diseases, Ankara University Medical School, Sancak Mahallesi 542, Sokak No. 6/4 Cankaya, Ankara, Turkey
Manuscript submitted October 4, 2017, accepted October 31, 2017
Short title: BCG Vaccine Complications
doi: https://doi.org/10.14740/ijcp279w
| Abstract | ▴Top |
Background: Bacille Calmette-Guerin (BCG) vaccine may cause some cutaneous complications at the injection site. Although some of these are normal reactions, unexpected complications may be seen. Clinical features, treatment modalities and outcomes of these complications have not been clearly defined in literature. We aimed to determine the local cutaneous complications after BCG vaccine and clinical experiences in our hospital.
Methods: Between May 2013 and December 2016, previously healthy children aged older than 2 months, without underlying disease or drug use history, who were admitted with localized cutaneous complications after BCG vaccine, were retrospectively analyzed. Demographic and clinical features, laboratory findings, treatments and outcomes were collected from database.
Results: Twenty patients were diagnosed as having local cutaneous complications. Abscess formation (30%), localized inflammation signs (45%), purulent discharge (10%), crust (10%) and eczematous lesion (5%) were found as local cutaneous complications. Drainage was performed in 14 (70%) patients. Oral amoxicillin clavunate therapy was given to four (20%) patients due to isolating Staphylococcus aureus (2/4), Staphylococcus hominis (1/4), and Exiguobacterium aurantiacum (1/4) from the drainage or purulent discharge culture. Anti-tuberculosis drugs (isoniazid and rifampicin) were used in six (30%) patients. Five of them had positive acid-resistant bacilli, tuberculosis polymerase chain reaction or tuberculosis culture results and one (5%) patient was diagnosed as having a lupus vulgaris because histopathological examination of his lesion showed granulomatous dermatitis. In addition, there was no lesion to drain in four (20%) patients and they were followed without treatment. On follow-up, all lesions completely recovered.
Conclusions: There is no still consensus about treatment of the local complication after BCG vaccine. In our study, some complications improved with conservative treatment. However, drainage, anti-tuberculosis drugs and antibiotic treatments were needed in some cases. As a result, treatment should be decided to each patient’s clinical symptoms and underlying disease.
Keywords: BCG vaccine; Clinical protocols; Infant; Local symptoms
| Introduction | ▴Top |
Mycobacterium tuberculosis remains an important problem worldwide [1]. Especially in developing countries with a high incidence of tuberculosis, the World Health Organization recommends that Bacille Calmette-Guerin (BCG) vaccine should be administered as soon as possible in all newborns [2]. BCG is a live attenuated Mycobacterium bovis vaccine which helps to protect children from meningitis and disseminated tuberculosis. However, this vaccine does not prevent primary M. tuberculosis infection or reactivation of latent tuberculosis [1].
In Turkey, 0.05 mL BCG strain has been administered by intradermal injection at all 2-month-old healthy infants. It is not given in younger than 2 months infants because of technical problems, high risk of complications and inadequate immune response. Some local cutaneous reactions may be seen in 2 - 3 weeks after vaccination. Pustules may occur at the injection site, and then they may be ulcerated. These lesions heal with scar in approximately 3 months. Although BCG vaccine complications are so rare, in literature erythema, soreness, abscess formation, purulent drainage, ulcer, keloid scar, blistering, regional lymphadenopathy, disseminated BCG-itis, osteomyelitis and osteitis have been reported in some cases [3-9]. There is no consensus on the treatment of local complications of BCG vaccine. In this study, we aimed to determine the local reactions after BCG vaccination and clinical outcomes in our hospital.
| Materials and Methods | ▴Top |
Our center is a tertiary care hospital which has 280-bed capacity and 215,000 pediatric patients have been admitted per year. Between May 2013 and December 2016, patients who were admitted with localized cutaneous complications of BCG vaccine were analyzed. Previously healthy children aged older than 2 months, without any underlying disease, history of illnesses or drug use, and abnormal findings who developed cutaneous lesions at the vaccinated area after BCG vaccination, were included in the study. Demographic and clinical features, personal and family history, laboratory findings, treatments and clinical outcomes were noted from database. Patients who were diagnosed as having congenital or acquired immune deficiency, disseminated BCG disease, lymphadenitis, osteitis or other BCG vaccine complications except cutaneous lesions and/or had underlying disease were excluded from the study. All patients received BCG vaccine at 2 months of age. Local cutaneous complications were identified as localized inflammation signs, abscess, purulent discharge, crust, lupoid reaction and eczema vaccinatum.
All patients were evaluated for immune deficiency. Analysis of lymphocyte subsets, serum immunoglobulins (Ig) G, A, M, E, complement 3, complement 4 and measuring the 50% hemolytic complement levels, T-cell receptor excision circles test, neutrophil oxidative burst test, lymphoblastic transformation test, CD212 expression of lymphocytes after 72 h culture with phytohemagglutinin and anti-human immunodeficiency virus (HIV) were performed.
Drainage was performed in all patients with abscess formation and localized inflammation signs, except one patient who had no lesion that could be drained, and only localized redness was present. All samples were stained with Erlich-Ziehl-Neelsen method to identify acid-resistant bacilli (ARB). In addition to abscess culture, tuberculosis polymerase chain reaction (PCR) and tuberculosis culture were performed from the drainage material. Tuberculosis skin test (TST) was made and chest X-ray was performed in all patients. When the microorganism was isolated from the drainage culture, appropriate antibiotics were used and at least one of the results of ARB, tuberculosis PCR and tuberculosis culture was positive, and the patient was treated with anti-tuberculosis treatment.
Ethics committee approval which included the principles of the Helsinki was taken.
Patients’ results were evaluated with the SPSS program and statistical analysis was expressed as number of observations (n), mean ± standard deviation (SD), median and minimum-maximum values M (min-max).
| Results | ▴Top |
In this study, 22 patients were diagnosed as having local cutaneous complications after BCG vaccine. However, two of them were excluded due to underlying immunodeficiency. Totally 20 patients without previously known disease or drug use story were included in the study. The overall annual incidence rate of BCG vaccine complications in the area that the hospital serves is detected as 1.33 per 100,000 pediatric population. The mean age was 6.6 ± 2.33 months. The mean development time of complications was 4.13 ± 1.97 months. Nine (45%) patients were boys. All patients did not have any family history. Anti-HIV test was negative and chest X-ray was normal in all children.
Abscess formation was detected in six (30%) patients. Drainage was performed in all these patients (Fig. 1). At least one of ARB strain, tuberculosis PCR or tuberculosis culture was positive in four (20%) of them who were treated with isoniazid and rifampicin for 3 months. Two (10%) patients were treated with oral amoxicillin-clavulanic acid for 10 days because of isolating Staphylococcus hominis in one patient and Exiguobacterium aurantiacum in the other patient from the drainage culture.
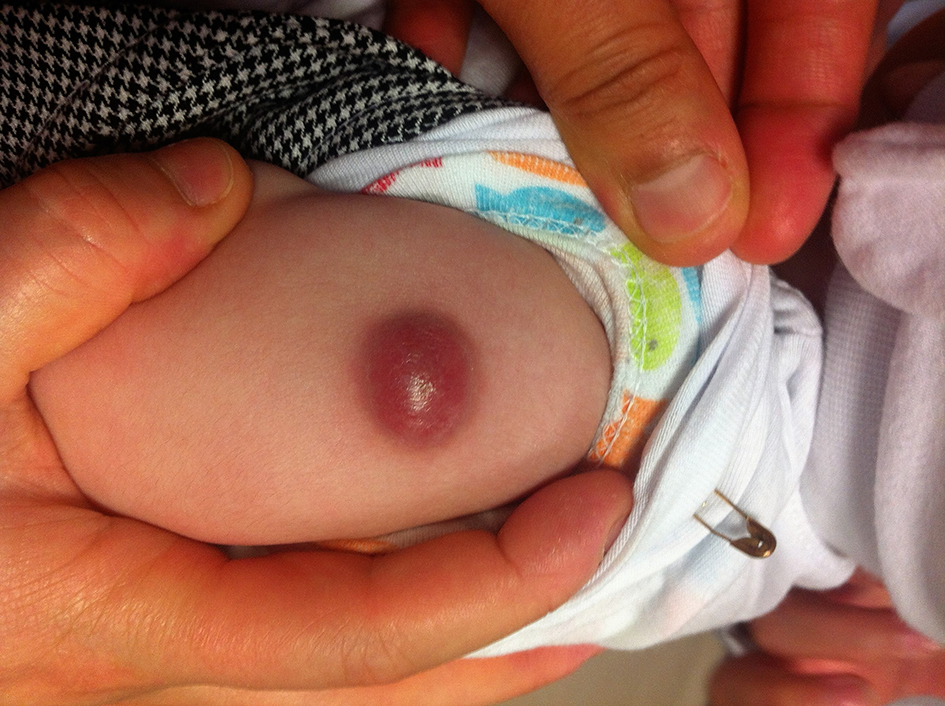 Click for large image | Figure 1. Localized abscess formation at the BCG vaccination site. |
Localized inflammation signs like hyperemia, swelling or heat increase at the injection site were detected in nine (45%) patients. Drainage was performed in eight of them. However, one patient with only localized hyperemia who had no lesion that could be drained was followed without treatment. ARB strain, tuberculosis PCR and tuberculosis culture were positive in one (5%) patient who was treated with isoniazid and rifampicin for 3 months.
Purulent discharge was detected in two (10%) patients. In all of them, S. aureus was isolated from swab culture and oral amoxicillin-clavulanic acid treatment was given for 10 days.
Eczematous lesion was detected in one (5%) patient who had positive TST (17 mm) result (Fig. 2). Histopathological examination of this lesion showed granulomatous dermatitis. The patient was diagnosed as having a lupus vulgaris. Thus, intravenous sulbactam ampicillin was given for 7 days. In addition, anti-tuberculosis drugs (isoniazid and rifampicin) were used for 6 months.
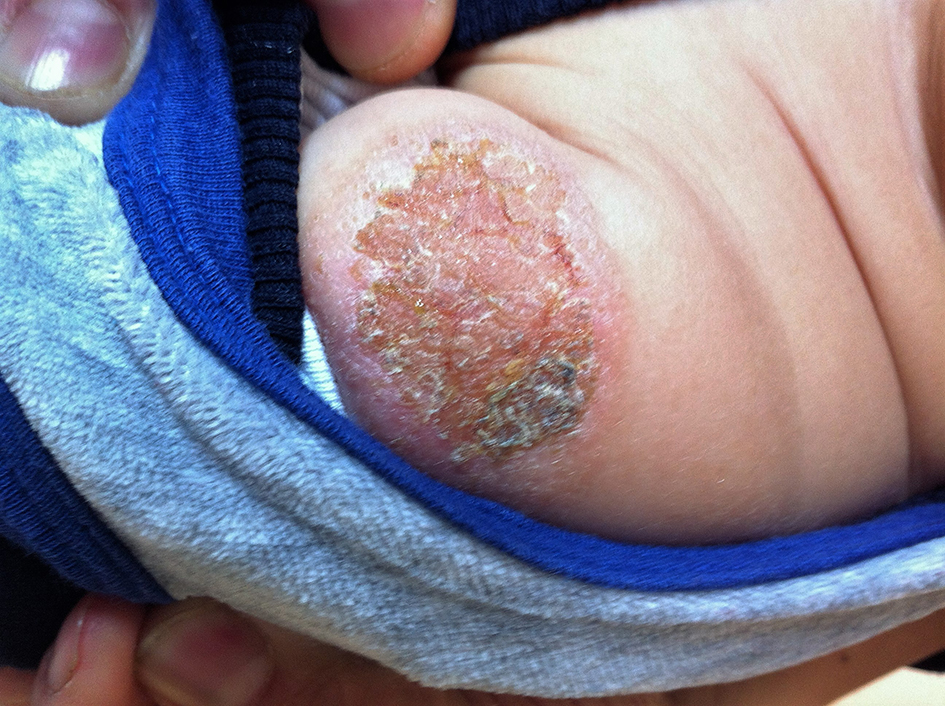 Click for large image | Figure 2. Eczematous locally squamous lesion at the BCG vaccination site. |
In addition to the above physical examination findings, no other abnormality such as lymphedema was found in the patients. Totally applied treatments were as follows: drainage in 14 (70%) patients, oral amoxicillin clavunate therapy in four (20%) patients, and anti-tuberculosis drugs in six (30%) patients. In addition, four (20%) patients were followed without treatment.
All patients were followed for 6 months and they completely recovered without complications. Clinical characteristics of patients and treatment modalities are summarized in Table 1.
 Click to view | Table 1. Clinical Characteristics of Patients With BCG Vaccine Complications and Treatment Modalities |
| Discussion | ▴Top |
In many countries, BCG vaccine is administered in routine immunization programs. Although it has been used in Turkey since 1952, single dose is applied in all healthy 2-month-old infants since 2006 [10]. Several complications have been reported after the routine immunization program of BCG vaccine. Although some of these are normal vaccine reactions, unexpected complications have been noted [3-9]. Adverse reactions may be affected with some factors like patient’s age, vaccine strains, dosage, erroneous administration, and disturbance of cellular immunity [11]. Some studies suggested that if the dosage of BCG vaccine is reduced, frequency of complications may be decreased [12, 13].
BCG vaccine can cause mild and severe complications. Mild complications include regional lymphadenitis, cutaneous lesions such as hyperemia, swelling, soreness, abscess formation, keloid and blister formation at the injection site, and eczema vaccinatum which are considered as normal vaccine reactions. Incidence of mild complications is estimated to be less than 1/1,000. Local ulceration at the vaccination site, suppurative lymphadenitis, osteitis, osteomyelitis, and disseminated BCG infection are severe complications which occur in approximately two cases per 1 million vaccinations [14]. Severe complications are more seen in patients with immunodeficiency disorders [15, 16]. Susceptibility to infections increases in patients with primary immune deficiency. Especially children with T cells deficiency have high risk for mycobacterial infections. Severe combined immunodeficiency (SCID), chronic granulomatous disease, complete DiGeorge syndrome, Mendelian susceptibility to mycobacterial disease and acquired immunodeficiency syndrome are some diseases which have increased risk for disseminated BCG infections [16, 17]. In addition, M. tuberculosis is an important cause of morbidity and mortality in HIV-infected children [16].
Treatment of cutaneous complications after BCG vaccine is unclear. The majority of reactions to the vaccine are localized and self-limited, and in most cases observation for 4 - 6 months is usually sufficient [2]. Although non-specific eruptions heal spontaneously, some complications require treatment. In our study, drainage (70%) was performed in patients who had abscess formation and localized inflammation signs. Amoxicillin clavunate (20%) was given to positive purulent discharge culture results. Anti-tuberculosis drugs were used in six (30%) patients. In addition, there was no lesion to drain in four (20%) patients and they were followed without treatment. The reasons why our results differ from the literature data are as follows: 1) most patients presented with abscess and localized inflammation; and 2) their parents were agitated, and drainage was performed early to obtain rapid clinical response.
Ying et al showed BCG complications were seen in more than 40% patients with primary immune deficiency [18]. Shahmohammadi et al reported primary immune deficiency was detected to be responsible for 59% of patients with disseminated BCG infection. Additionally SCID was the most causative immune deficiency for BCG vaccine complications [7]. Marciano et al reported after vaccination various BCG associated manifestations were seen in half of the patients with SCID. Localized disease occurred in one-third of patients and disseminated disease was seen in two-thirds [3]. In the above studies, primer immunodeficiencies were detected frequently causative for BCG complications. Our results were different, because the patients with immunodeficiency were excluded. In the literature, many studies about the treatment of BCG vaccine complications in immunocompromised patients have been reported. However, there are limited data on the approach in healthy children. For this reason, we aimed to determine our clinical experience in BGC vaccine reactions in healthy children and we excluded immunosuppressed patients from this study.
After BCG vaccination, several complications are reported in literature. Venkataraman et al found between January 2008 and December 2013, 60 children presented with adverse vaccine reaction. Isolated injection site reactions occurred in 18 (30%) children and isolated axillary lymphadenitis was detected in 39 (65%) children. All patients with isolated injection site reactions were treated as follows: 16 (88%) conservative therapy, one (6%) anti-tuberculosis treatment and one (6%) only aspiration. Seventeen (95%) patients completely recovered. One patient was admitted to hospital again because of swelling at the injection site. After total excision, the lesion healed with scar. As a result, “watch and wait” was recommended for isolated injection site reactions [19].
Daoud showed regional lymphadenitis (61.3%), local abscess (21.3%), local ulcer (11.5%), keloid scar (5.3%) and disseminated disease (0.4%) were found as complications after BCG vaccine [20]. Our patients did not have any localized lymphadenopathy or disseminated BCG disease. We believe that this difference may be explained with the fact that the patients who had only cutaneous lesions were included.
Treatment of cutaneous complications after BCG vaccine is unclear. In literature anti-tuberculosis drugs are not recommended for routine treatment of local complications. Low penetration of anti-tuberculosis drugs into the abscess cavity and pyrazinamide resistance of M. bovis strains are some problems [5, 21]. Early drainage is recommended for abscess formation and suppurative lymphadenitis [5]. Needle aspiration of abscess helps rapidly recovery. Although most infants recover with one aspiration, repeated aspiration may be needed [22].
Cuello-Garcia et al found isoniazid injection after aspiration may be useful, but there are limited data in literature [5]. In our study, oral isoniazid and rifampicin treatments were given to six (30%) patients. One of them had histopathology findings such as granulomatous dermatitis. Five of them had positive at least one of ARB, tuberculosis PCR or tuberculosis culture results. We believe that this positivity was caused by M. bovis as well, but we could not identify the type of M. tuberculosis complex. The patients were treated with anti-tuberculosis drugs to obtain rapid clinical response for 3 months. All patients completely recovered without complications.
Lupus vulgaris is a common clinical type of cutaneous tuberculosis. Although it is rare, it may be seen after BCG vaccination. Anti-tuberculosis drugs are used for treatment [23]. In our patient with lupus vulgaris, clinical findings recovered with isoniazid and rifampicin treatments.
There were some limitations in our study. Few patients were included. In addition, we obtained different results from the literature because the patients with immunodeficiency were excluded from our study. The approach to BCG vaccination reactions is clearly known in immunocompromised children. However, in healthy children, there were limited data in the literature.
Conclusion
BCG vaccine may cause some adverse reactions at the vaccination site. Hyperemia and swelling, heat increase, abscess formation, crust, lupoid reaction, and eczema vaccinatum may be seen as complications. While reactions are usually mild, serious complications may also occur. Although most complications improve with conservative treatment, suppurative lymphadenitis, localized abscess and localized inflammation formation mostly require drainage. Anti-tuberculosis treatment or antibiotic drugs are necessary in some cases with positive drainage culture, ARB, tuberculosis PCR or tuberculosis culture results. Although cutaneous complications of BGC vaccine may be various, underlying diseases and complications should be the most important guides for treatment.
Funding Source
No grant, honorarium, or other form of payment was given to authors to produce this manuscript.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
Disclosure
The authors have nothing to disclose.
| References | ▴Top |
- World Health Organization, and World Health Organization. Management of Substance Abuse Unit. Global status report on alcohol and health, 2014. World Health Organization, 2014.
- World Health O. BCG vaccine. WHO position paper. Wkly Epidemiol Rec. 2004;79(4):27-38.
- Marciano BE, Huang CY, Joshi G, Rezaei N, Carvalho BC, Allwood Z, Ikinciogullari A, et al. BCG vaccination in patients with severe combined immunodeficiency: complications, risks, and vaccination policies. J Allergy Clin Immunol. 2014;133(4):1134-1141.
doi pubmed - Keijsers RR, Bovenschen HJ, Seyger MM. Cutaneous complication after BCG vaccination: case report and review of the literature. J Dermatolog Treat. 2011;22(6):315-318.
doi pubmed - Cuello-Garcia CA, Perez-Gaxiola G, Jimenez Gutierrez C. Treating BCG-induced disease in children. Cochrane Database Syst Rev. 2013;1:CD008300.
doi - Mahmoudi S, Khaheshi S, Pourakbari B, Aghamohammadi A, Keshavarz Valian S, Bahador A, Sabouni F, et al. Adverse reactions to Mycobacterium bovis bacille Calmette-Guerin vaccination against tuberculosis in Iranian children. Clin Exp Vaccine Res. 2015;4(2):195-199.
doi pubmed - Shahmohammadi S, Saffar MJ, Rezai MS. BCG-osis after BCG vaccination in immunocompromised children: case series and review. J Pediatr Rev. 2014;2(1):47-54.
- Lin WL, Chiu NC, Lee PH, Huang AS, Huang FY, Chi H, Huang DT, et al. Management of Bacillus Calmette-Guerin osteomyelitis/osteitis in immunocompetent children-A systematic review. Vaccine. 2015;33(36):4391-4397.
doi pubmed - Bellet JS, Prose NS. Skin complications of Bacillus Calmette-Guerin immunization. Curr Opin Infect Dis. 2005;18(2):97-100.
doi - Ozmert EN. Dunya'da ve Turkiye'de asilama takvimindeki gelismeler. Cocuk Sagligi ve Hastaliklari Dergisi. 2008;51(3):168-175.
- Bolger T, O'Connell M, Menon A, Butler K. Complications associated with the bacille Calmette-Guerin vaccination in Ireland. Arch Dis Child. 2006;91(7):594-597.
doi pubmed - Krepela V, Galliova J, Kubec V, Marik J. [The effect of reduced doses of BCG vaccine on the occurrence of osseous complications after vaccination]. Cesk Pediatr. 1992;47(3):134-136.
pubmed - Teulieres L, Diouf MA, Chaud P, Saint-Cyr A, Saliou P. Comparative trial of administration of half (0.05 mg) and quarter (0.025 mg) dose of intradermal Pasteur BCG on 291 infants from birth to 1 year in French Guyana. Vaccine. 1991;9(7):521-524.
doi - Govindarajan KK, Chai FY. BCG Adenitis-Need for Increased Awareness. Malays J Med Sci. 2011;18(2):66-69.
pubmed - Sadeghi-Shabestari M, Rezaei N. Disseminated bacille Calmette-Guerin in Iranian children with severe combined immunodeficiency. Int J Infect Dis. 2009;13(6):e420-423.
doi pubmed - Boisson-Dupuis S, Bustamante J, El-Baghdadi J, Camcioglu Y, Parvaneh N, El Azbaoui S, Agader A, et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev. 2015;264(1):103-120.
doi pubmed - Al-Hajoj S, Memish Z, Abuljadayel N, AlHakeem R, AlRabiah F, Varghese B. Molecular confirmation of Bacillus Calmette Guerin vaccine related adverse events among Saudi Arabian children. PLoS One. 2014;9(11):e113472.
doi pubmed - Ying W, Sun J, Liu D, Hui X, Yu Y, Wang J, Wang X. Clinical characteristics and immunogenetics of BCGosis/BCGitis in Chinese children: a 6 year follow-up study. PLoS One. 2014;9(4):e94485.
doi pubmed - Venkataraman A, Yusuff M, Liebeschuetz S, Riddell A, Prendergast AJ. Management and outcome of Bacille Calmette-Guerin vaccine adverse reactions. Vaccine. 2015;33(41):5470-5474.
doi pubmed - Daoud W. Control of an outbreak of BCG complications in Gaza. Respirology. 2003;8(3):376-378.
doi pubmed - Hesseling AC, Schaaf HS, Hanekom WA, Beyers N, Cotton MF, Gie RP, Marais BJ, et al. Danish bacille Calmette-Guerin vaccine-induced disease in human immunodeficiency virus-infected children. Clin Infect Dis. 2003;37(9):1226-1233.
doi pubmed - Riordan A, Cole T, Broomfield C. Fifteen-minute consultation: Bacillus Calmette-Guerin abscess and lymphadenitis. Arch Dis Child Educ Pract Ed. 2014;99(3):87-89.
doi pubmed - Selimoglu MA, Erdem T, Parlak M, Esrefoglu M. Lupus vulgaris secondary to single BCG vaccination. A case report. Turk J Pediatr. 1998;40(3):467-471.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
